Thin Layer Chromatography (TLC) for the Separation of Lipids
Background
Thin layer chromatography (TLC) is a tried and true method for the separation of components in a mixture based on the polarity of the individual components. When a standard is included, this method can also be used for the identification of each component of the mixture.
The TLC technique requires 2 phases: a stationary phase, which involves some sort of matrix, and a mobile phase, which is a mixture of solvents. The type of mixture you are interested in separating dictates the precise materials used for the 2 phases.
The stationary phase for TLC consists of a plate, usually a sheet of plastic or glass, that is coated with an absorbent material (i.e. silica). The extracted sample mixture (such as a lipid extract from cells) is applied at the bottom of the plate, allowing the solid phase to capture the mixture. The plated sample is then “developed” by placing the TLC plate into a sealed chamber containing the mobile phase solvent system. The solvent system is selected based on its ability to dissolve the desired components to be separated. This chamber also contains a Whatman Paper “wick,” which is a sheet of filter paper that helps to ensure that the chamber is evenly saturated with solvent vapors.
As the solvent migrates up the plate, it will carry with it the different components of the mixture. The more soluble the components in the solvent, the easier it will migrate up the plate. Also affecting how the samples migrate is the absorbent material on the plate. For instance, silica, which is polar, will strongly interact with the polar components of the mixture. Therefore, polar components will “stick” to the silica, and will migrate up the plate with less efficiency than components that are weakly polar or nonpolar. In general, as the polarity of a component (molecule) decreases, the ability for it to move up the plate increases.
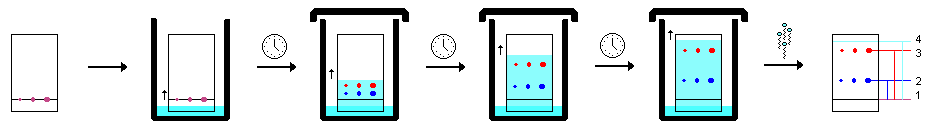
TLC Sequence
For the purposes of lipid separation, particularly for neutral lipids such as triglycerides, we will be using a silica coated plastic plate (stationary phase) and an organic, largely nonpolar solvent mobile phase consisting of petroleum ether: diethyl ether: acetic acid at a ratio of 84:15:1. The lipids are then visualized using resublimed iodine, which will bind to double bonds found in lipid hydrocarbon chains and aromatic compounds.
As summarized in the table below, this solvent mixture is intended to capture both nonpolar and polar species in the lipid extract. The polarity index for petroleum ether (0.1) positions this solvent component as extremely nonpolar, and will allow the most nonpolar lipid in the mixture to be “dissolved.” Whereas acetic acid, which has a high polarity index (6.2), is much more polar since it has the capacity to ionize, and serves as a solvent for the more polar species in this mixure. However, do note that the components in this solvent are not provided in equal quantities; it is both the polarity index as well as the relative amount of each solvent component that dictates how it will carry specific lipid species up the TLC plate.
| Solvent Component | Polarity Index | Ratio |
| Petroleum Ether | 0.1 | 84 |
| Diethyl Ether | 2.8 | 15 |
| Acetic Acid | 6.2 | 1 |
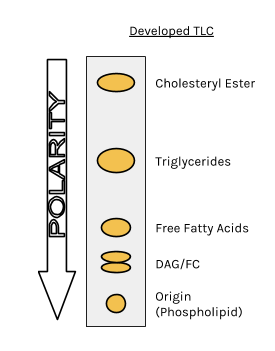
The less polar a component in a mixture is, the further it will move up on the TLC plate.
Materials

Chemicals
- Lipid Extract
- Petroleum Ether*
- Diethyl Ether*
- Glacial Acetic Acid*
- Lipid Standard
- Resublimed Iodine*

Specialty Equipment
- Silica-coated TLC plates (these can be purchased from Sigma Aldrich or other similar scientific suppliers)
- Whatman Paper “wick”
- TLC Chamber (can use ball jars)
- Pipette Tips or Glass Capillary Tubes
Lab Equipment
- Micropipette (can use glass Pasteur pipette)
- Hotplate
- Fume Hood
- Pencil
- Lab tape
Safety
*Note: Indicated items should be used in a fume hood, and must be handled with the appropriate personal protective equipment, including gloves and safety goggles.
Preparation
- Prepare the TLC chamber, which can be as simple as a mason jar, with the solvent system.
- Pre-cut the wick such that it is a little bigger than the TLC plate and insert into TLC chamber.
- Close the lid to the TLC chamber and allow to equilibrate for about 10 minutes or more.
- Turn on hot plate to a low setting.
- Prepare visualization chamber by placing some resublimed iodine crystals into a mason jar and secure the lid.
Procedure
- Using 2 small pieces of tape, attach the TLC plate to the hotplate such that the bottom of the plate is touching the hotplate.
- Mark the origin (bottom) of your plate with a pencil and label each lane.
- Slowly spot the standard in the first lane in increments of 5 μL, allowing the spot to dry.
- Repeat 3 times for a total of 15 μL standard.
- Next, slowly spot the lipid extract in the designated lanes in increments of 5 μL, allowing the spots to dry.
- Repeat 5 times for a total of 25 μL sample.
- If you do not have micropipettes, you can use a glass Pasteur pipette to add your lipid extract to the plate in a dropwise fashion, being sure that you allow the “spot” to dry in between each drop
- Place the plate into the charged TLC chamber, in front of the wick.
- Secure the lid to the chamber and allow the plate to develop.

- Once the solvent front nears the top of the plate, remove the plate from the TLC chamber, quickly mark the solvent front with a pencil, and allow it to dry.
- It typically takes 30-60 minutes for the solvent front to reach near the top of the plate.
- Place the plate into the iodine chamber for visualization and secure the lid.
- Once adequately stained (about 3-5 minutes), remove and lightly outline the spots and/or take a picture.
What conclusions can you draw about the polarity of the substances?
Discuss one purpose of this technique in research or industry.