Cooking Eggs with Acid
Background
Eggs are a nutritious source of protein. The egg white is largely made up of protein, second only to the water content in the white (87% water, 11% protein by mass). Egg yolks also contain significant amount of protein (52% water, 16% protein by mass), but also significant amounts of lipids (27% lipids by mass). Overall an egg is just under 13% protein by mass. (You can visit USDA’s National Nutrition Database for more details.)
Cooking an egg (or many other protein-rich foods) largely involves denaturing the proteins, and cross-linking them with each other to form the rubbery, white substance we are familiar with eating. We think of cooking using heat; however, the proteins can also be denatured with a strong acid to disrupt the intermolecular forces chemically. This is the approach we will use today.
Materials

Corner Store Items
- Egg Whites

Lab Chemicals
- 6M HCl
Lab Items
- Glass petri dish, beaker, or jar (glass is best when working with strong acids)
- Micropipette and Micropipette Tips (if you do not have access to a micropipette, you can use a glass Pasteur pipette with a rubber bulb)
Safety
The egg white should be in a glass container as to not risk unfavorable reactions between strong acids and some plastics. Always exercise caution when working with strong acids, and wear the appropriate personal protective equipment while handling strong acid (which should include safety goggles). If passing the sample around to students, ensure the sample is securely closed or that students are also wearing personal protective equipment.
Procedure
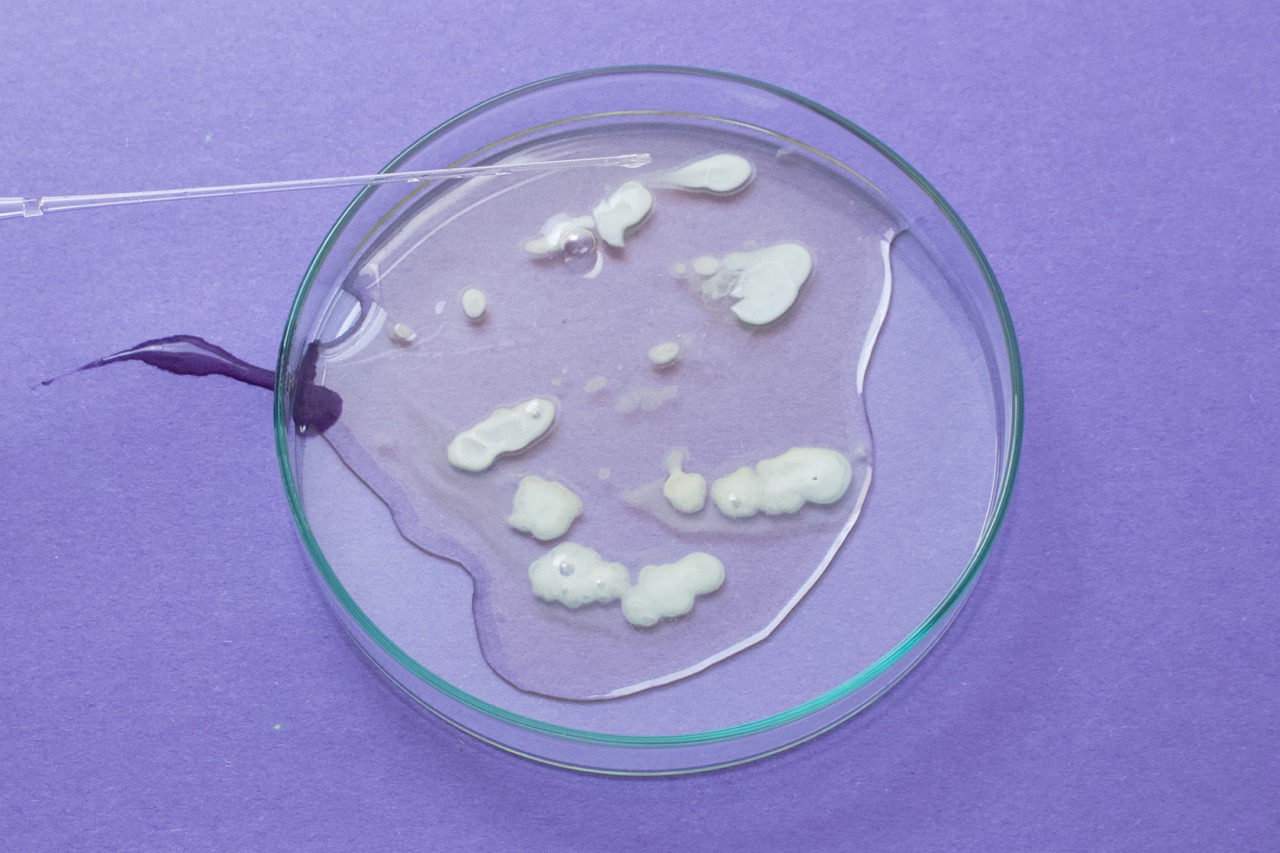
- Begin with 3.0 g egg white in a small glass container; ideally one with a lid for safe observation by students
- Add 6M HCl dropwise into the eggwhite. If using a micropipette, you may want ~0.5 mL. Be careful of drips as you transfer strong acid.
- Swirl slightly to observe the chemical changes happening over time. Go slowly and observe as you add the acid.
- Cover and continue to observe the sample over the next half hour. Eventually, you can poke gently with a pipette tip to study the final texture of your egg white.
Variations
This experiment can be done with acids of varying concentration, including distilled white vinegar that you probably have in your kitchen. Weaker acids will take longer to cause visual changes.
Discussion
The acid is affecting the proteins in the egg white. Is the acid breaking bonds or IMFs? Is it forming bonds or IMFs? How do you know?
What conclusions can you draw about how acid affects protein structure?