Know That Science is LIT
Deepen your knowledge...
Electromagnetic Radiation
There are two basic types of waves: compressional and transverse waves. Compressional (e.g. sound) waves require a medium to travel, while transverse (e.g. light) can travel in a vacuum and are faster. This is why light travels faster than sound (think of thunderstorms: you see lighting before hearing thunder).
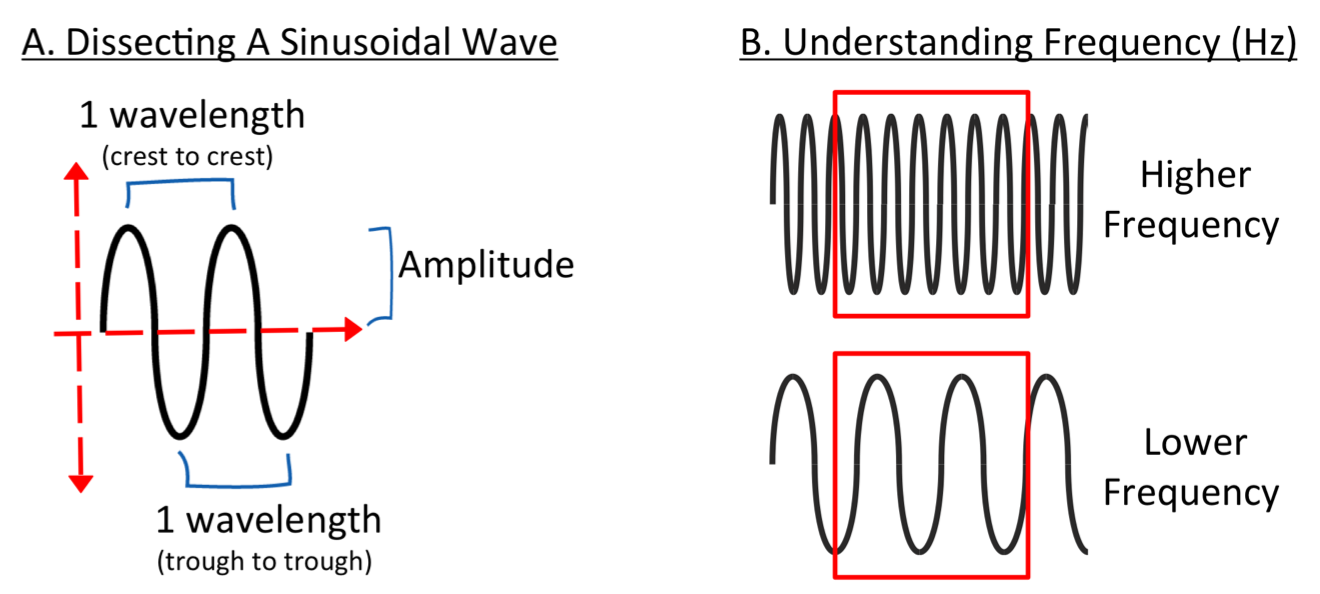
Electromagnetic radiation moves in a sinusoidal wave-like manner and is described by the properties of the wave. The length from the one crest (top) to crest or trough (bottom) to trough is considered 1 wavelength and is described in meters. The height from the center of the propagating wave to the top or bottom of the wave is called the amplitude. The amplitude tells us how much energy the wave has (for example think of a small wave at the beach compared to a tsunami) and it is measured in meters as well. The amplitude of a particular wavelength of light is a measure of the intensity or brightness of it. Another descriptor of electromagnetic radiation is frequency, which is the number of waves that pass through a certain point in a certain amount of time. Frequency is measured in hertz (Hz) after Heinrich R. Hertz who showed that light is a form of electromagnetic radiation. Hertz is defined as one cycle per second.
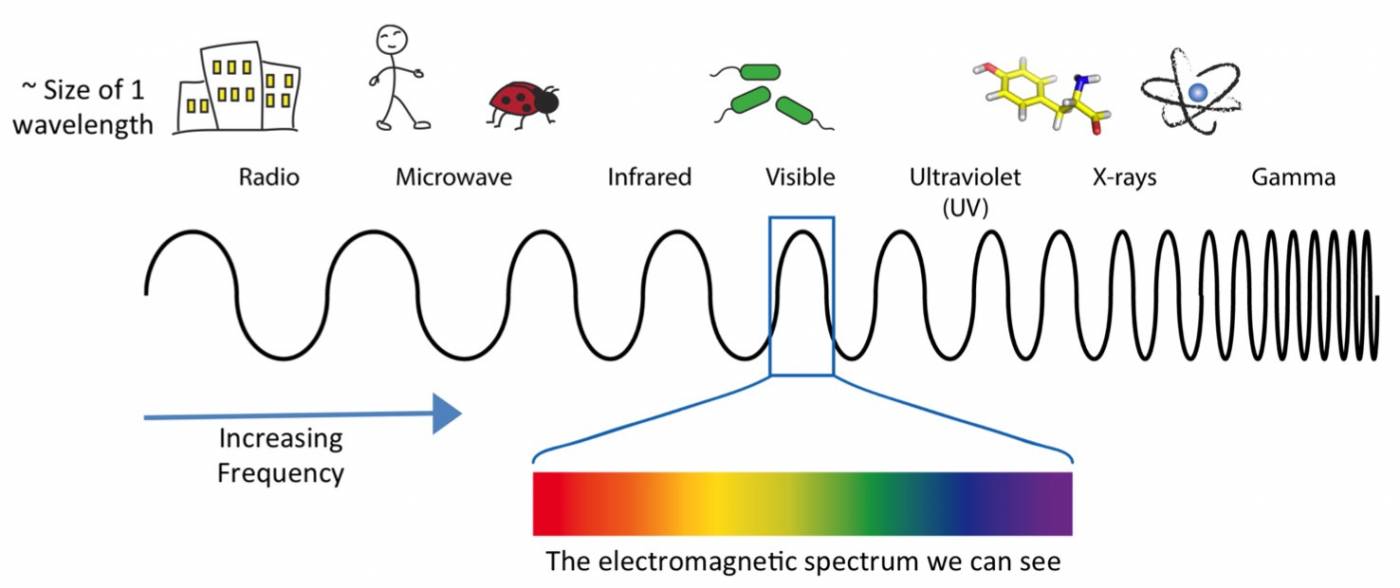
Electromagnetic waves fall on a spectrum of long to short wavelengths. Different wavelengths have different levels of energy associated with them. Shorter wavelengths have higher energy (e.g. x ray), and longer wavelengths have lower energy (e.g. radio waves). This make sense if you consider how frequent shorter wavelengths are in a defined time compared to longer wavelengths.
Light Interacting with our Environment
Light interacting with our environment
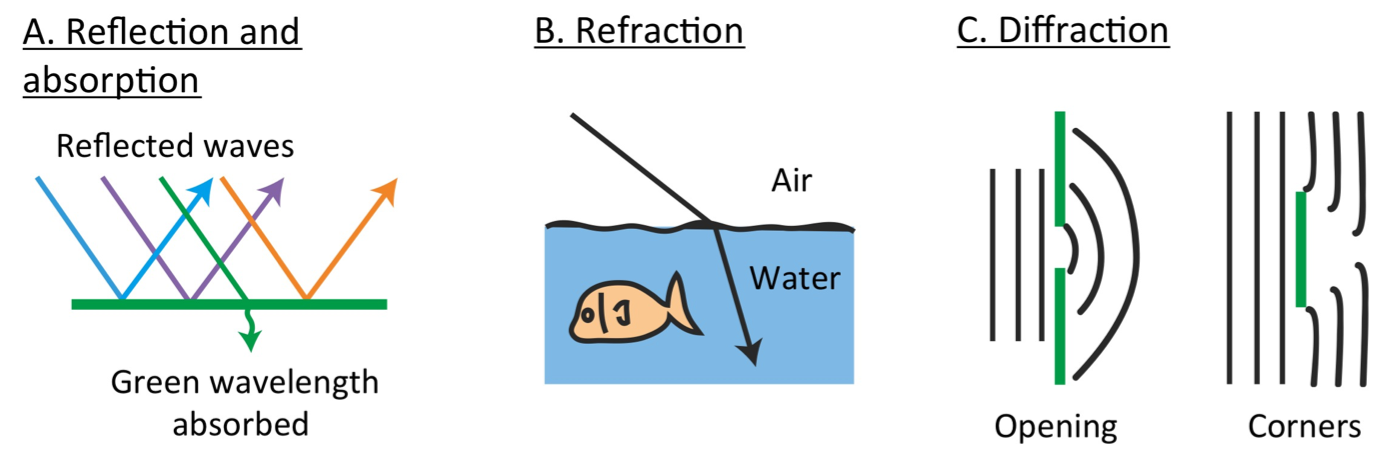
Light/electromagnetic radiation travels in a straight line and can do a couple of things when it hits an object. If we think about a specific wavelength of light as it interacts with an object, it can be:
- Reflected (reflection) – electromagnetic radiation/light bounces off the surface of the object. In order to see an object that doesn’t make it’s own light like the sun, light needs to bounce off the object and reach our eyes.
- Absorbed (absorption) – energy from the electromagnetic radiation/light is taken up/‘soaked up’ by the object (provide Ex.)
- Whether light is absorbed depends on the wavelength of light and atoms that the object is made up of. Different atoms or molecules have specific vibrational frequencies and when the vibrational frequency of the molecule matches the wavelength of light that hits it, the light gets absorbed.
- Transmitted (transmission) – when the light moves through the object.
- Diffracted (diffraction) – the spreading/bending of light as it goes through openings or around the edges of objects. The spreading of electromagnetic radiation looks a lot like the ripples you make in water when you dip your toe in.
- If the wavelength of the electromagnetic radiation is much smaller than the object around which is bends or opening it is going through, then very little to no diffraction is observed.
- Refracted (refraction) – the bending of transmitted light as it travels across the boundary of one material into another material in which it’s speed is different. Unlike diffraction, this change in direction of light occurs because light is changing its speed in the different substances in it traveling in.
- The refractive index of a material is a number that describes how light will travel in it compared to how it travels in air or in a vacuum. The higher the refractive index of a substance, the slower light travels.
Something to keep in mind: We learnt earlier that the visible white light we see actually composed of different wavelengths. Therefore, when white light hits/interacts with objects, what happens to the light is complex/complicated with some of the wavelengths reflected while others are absorbed or transmitted.
Sunlight that reaches our atmosphere is composed of all the colors of the rainbow (ROYGBV). The different wavelengths of light are scattered differently by particles in the air. Smaller wavelengths scatter more than longer wavelengths.
Dyes and Pigments
Background
Different molecules absorb different wavelength of electromagnetic radiation, and reflect the remaining one, which is the color we see. For example, chlorophyll, a pigment found in many plant leaves absorbs blue and red light, reflecting green light back into our eyes, making plants appear green. Another illustration of absorption are sunglasses – they only let a small proportion of the electromagnetic radiation from the sun pass through the lenses into our eyes, protecting them from harmful UV radiation and intense light. In addition to absorption, most sunglasses also reflect light, which also keeps it out of our eyes.

Hydrangeas can change color from pink to blue depending on the pH of their soil
The structure of a molecule determines the wavelengths it absorbs. The part of the molecule responsible for the color it appears, as a powder or liquid, is called the chromophore. The structure of a molecule can change as the pH changes e.g from acidic to alkaline, and this can include the chromophore. Substances that change color as the pH changes are useful as indicators and can be used in the lab to determine if a substance is acidic or basic or neutral. For example, litmus, a compound found in lichen, is purple under neutral conditions, but turns red in acidic conditions and blue in alkaline conditions.
Why are leaves green?
Plant cells contain chloroplasts, and chloroplasts contain chlorophyll, a green pigment. There are other pigments in plant cells, but much less than the chlorophyll.
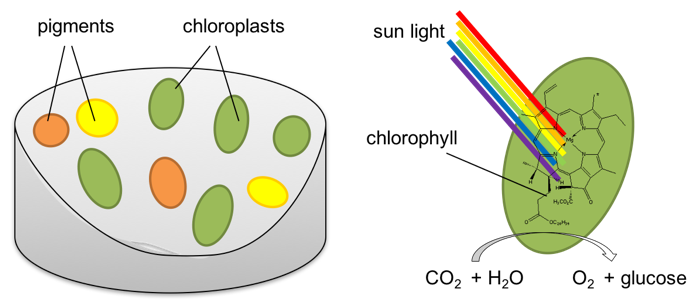
Chlorophyll absorbs light in the blue and red region of the electromagnetic spectrum and plants use the energy from the light they absorb to catalyze a reaction of carbon dioxide and water to form oxygen and glucose, this is called photosynthesis. They release the oxygen into the air and use the glucose, a sugar, as food.