Single Step Lipid Extraction From Food Stuffs
Background
Total lipid extraction from biological samples is a commonly used technique to assess the individual lipids that make up a tissue. One of the best described methodology for lipid extractions remains the Folch Extraction, named for the protocol’s author, Jordi Folch, who outlined this procedure in a seminal paper published in 1957. It very quickly became one of the most widely used protocols of its time, and is still the gold standard against which all other lipid extraction protocols are judged.
The premise of this technique is to first suspend homogenized tissues in a single phase (monophasic system) by the addition of chloroform:methanol mixture (2:1, v/v). This solvent mixture is chosen because it is moderately polar (see Polar Index below), which is helpful since the cells that make up the tissue are mostly made up of water — you want to be able to maximize how much of the polar and nonpolar components that can be dissolved (aka perturbation of intermolecular forces).
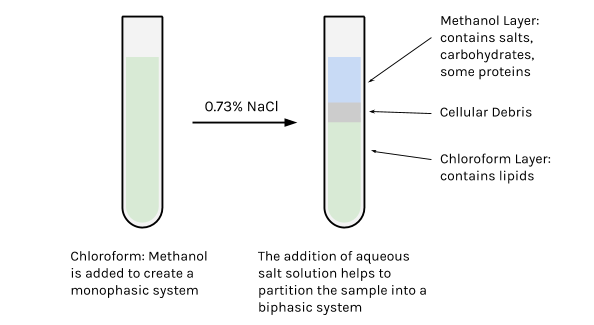
At this point in the extraction, the lipid sample is not pure. Many of the lipids are still strongly associated with non-lipid components such as proteins and carbohydrates (polysaccharides), reflecting their biological roles in cells. These “contaminants” can be removed by the addition of an aqueous solution (NaCl or KCl, for example), which helps the mixture to partition into 2 phases (biphasic system). The lipids are drawn into the chloroform portion, which is the least polar and most dense, and represents approximately 60% of the total volume. The upper phase, which is mostly methanol and water, contains mostly the non-lipid contaminants (though a residual amount of the more polar lipids might be present).
In this protocol, we have adapted the Folch Extraction to isolate lipids from common lipid-rich foods: avocados, eggs, and mayonnaise. These lipids can then be separated by thin layer chromatography, giving us insights into the lipids found in each ingredient.
| Reaction Component | Density | Polarity Index* |
| Chloroform | 1.49 g/cm3 | 4.1 |
| Methanol | 0.79 g/cm3 | 5.1 |
| 0.73% NaCl (H2O) | 1.01. g/cm3 | 10.2 |
*The Polarity Index is a measurement of how reactive the solvent is with various polar test solutes. The higher the polarity index, the more polar the solvent.
Materials
Foods
Any assortment; make sure at least some contain substantial fat content. E.g.
- Ripe Avocado
- Egg Yolk
- Egg White
- Mayonnaise
Solvents / Solutions
- Chloroform*
- Methanol*
- 0.73% NaCl Solution
*Note: Chloroform and methanol should be used in a fume hood, and must be handled with the appropriate personal protective equipment, including gloves and safety goggles.

Materials for Sample Preparation
- 15mL Conical Tubes and/or Glass Tubes
- Paper Cups for manipulating food samples
Note: Lipid preparations are best done in glass since plastic materials can affect lipid extractions — glass or Teflon-coated plastics are preferred. However, this is only critical when performing analytical assays or assays that have a high sensitivity.
Equipment
- Mortar and Pestle to grind solid foods
- Spoon or Spatula
- Glass Pasteur Pipettes & Rubber Pipette Bulbs
- Rack for test tubes or similar
- Centrifuge (if you do not have a centrifuge, see note under step 7 of protocol)
- Micropipettes & Tips (if you do not have access to micropipettes, you can still perform this protocol using glass Pasteur pipettes)
Preparation
Many of the consumables listed in this protocol are reusable. Furthermore, it should be noted that lipid preparations are best done in glass since plastic materials can affect lipid extractions — glass or Teflon-coated plastics are preferred. However, this is only critical when performing analytical assays or assays that have a high sensitivity.
- Using a glass bottle, prepare the chloroform:methanol solution such that it is 2:1 v/v.
- Store in appropriate location as per lab safety instructions.
- Work in a fume hood.
- Use the mortar and pestle to mash avocado flesh such that it is a smooth and velvety paste.
- Crack the egg and separate the white and yolk into different paper cup containers.
- Have mayonnaise on hand.
- Label tubes to appropriately reflect contents.
- Transfer ~2g of individual food material to each tube.
Procedure
- To each tube of foodstuffs, add 1 mL of chloroform:methanol (2:1, v/v) solution.
- Secure the cap on the tube and vigorously shake the samples for 30 seconds.
- Place the tubes back into a rack, and remove the caps.
- At this point, you have created a monophasic system whereby components are dissolving in the solution.
- Add 267 μL of the 0.73% NaCl solution to each tube to make a chloroform: methanol: water mixture (2:1:0.8, v/v/v).
- Secure the caps back onto the tubes and again shake vigorously for 30 seconds.
- The addition of the aqueous salt solution transforms the mixture into a biphasic system, and helps to separate the hydrophobic lipid components from water-soluble (hydrophilic) components.
- Because chloroform is more dense than methanol and water, the chloroform-lipid phase will move toward the bottom of the tube, and on top of this will sit the less dense methanol-salt phase (thereby creating a biphasic system).
- Spin conical tubes in a benchtop centrifuge at 2,500 rpm for 2 minutes.
- Carefully remove the tubes from the centrifuge, being sure not to disturb the phase separation.
- If you do not have a benchtop centrifuge, you can allow the tubes to sit, undisturbed, for 30-60 minutes, which will allow the phases to completely separate.
- Transfer the top, methanol-water phase, to a waste container labeled for methanol disposal.
- If you have interest and ability, this phase can be used to assess non-lipid components of the food materials.
- Label a fresh glass tube with the appropriate food label.
- Using a glass pipette, transfer the bottom, chloroform-lipid phase to this freshly labeled glass tube.
- There will still be a small amount of biomass in the tube, above the chloroform-lipid phase — try to get to the chloroform-lipid phase without disturbing the biomass too much. You can just quickly “push” your glass pipette tip through the biomass to get to the bottom.
What observations can you make about the lipid samples?
- The lipid is now ready to be analyzed using TLC.
Discussion
Chloroform is relatively nonpolar while methanol and water are more polar. Methanol and water are similar enough in polarity that they can mix. However, the added charges from the dissolved salt shift this dynamic in favor of separate phases, where the polar and charged molecules are drawn into the polar solvent layer.
Are the molecules that add color (e.g. the “green” in avocado) hydrophilic or hydrophobic?
Students may be curious that the nonpolar layer is on the bottom since they are familiar with oil floating on water. Remind them that density is a property of different substances that is independent of polarity or intermolecular interactions.
Cells are made of lots of different molecules closely packed together, and in some cases even bonded together. If these bonds and intermolecular interactions are not sufficiently disrupted, they stay in large clumps. Some large molecules with complex behaviors also reside here (e.g. DNA). Here we refer to all of this as “cellular debris.”
Most nonpolar solvents are relatively hazardous if we get them on our skin. Why do you think this would be?
Remember that our skin is made up of a sturdy barrier of lipids. As shown in this extraction, a nonpolar solvent can dissolve the lipids from our cells, breaking down this strong barrier. This means that some of these nonpolar solvent molecules could get through our membranes into our bodies, or worse, they could dissolve pieces of membrane or whole cells.