Oil and Water
Background
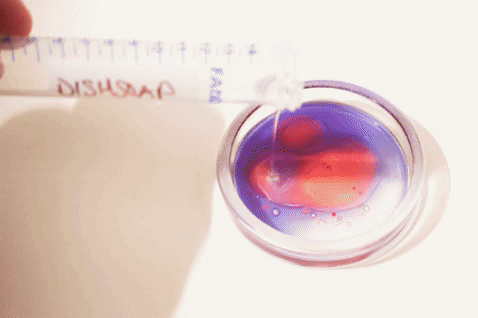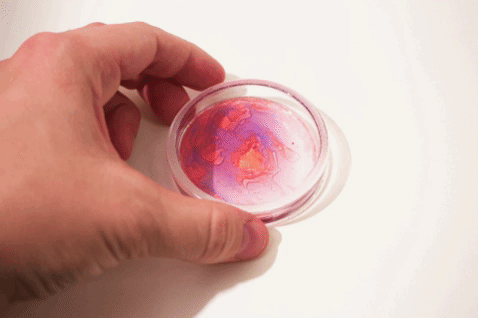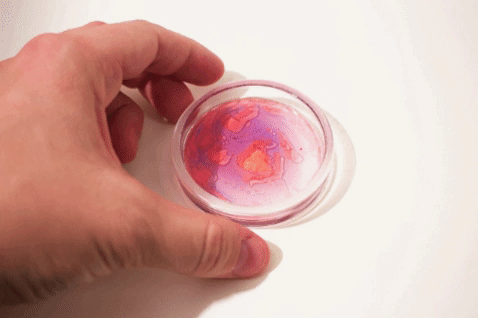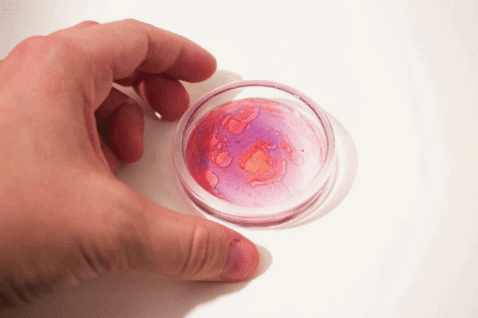
We’ve all heard that oil and water don’t mix, but what does that mean and how can we study more complex behaviors like emulsions?
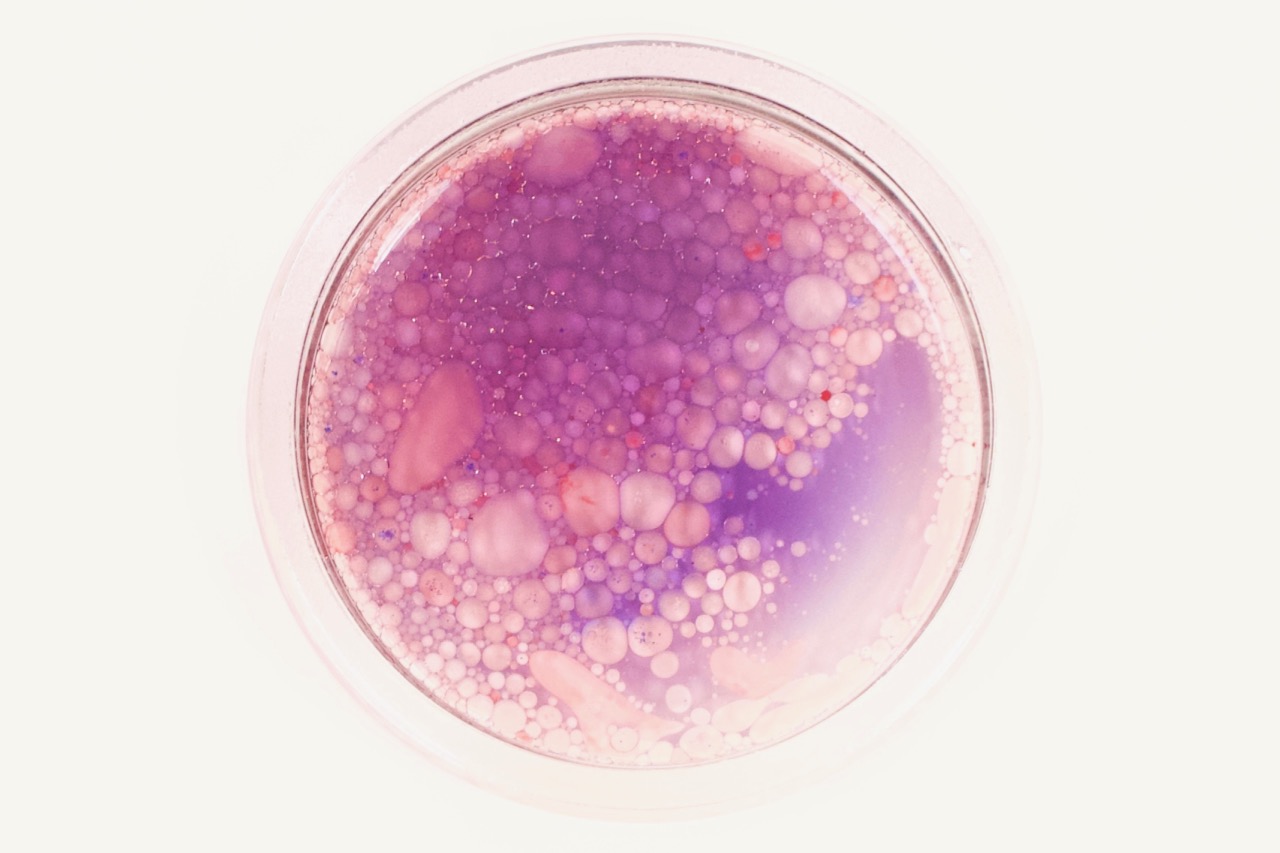
Luckily, just as most molecules have hydrophilic or hydrophobic preferences, various dye molecules (molecules that have color) can also have hydrophilic or hydrophobic preferences. Some dyes prefer to hang out with the water, like food coloring. Other dyes like to hang out with the lipids, like a special (but inexpensive!) dye called Oil Red O. These can allow us to explore these intermolecular interactions and phase separations in colorful detail.
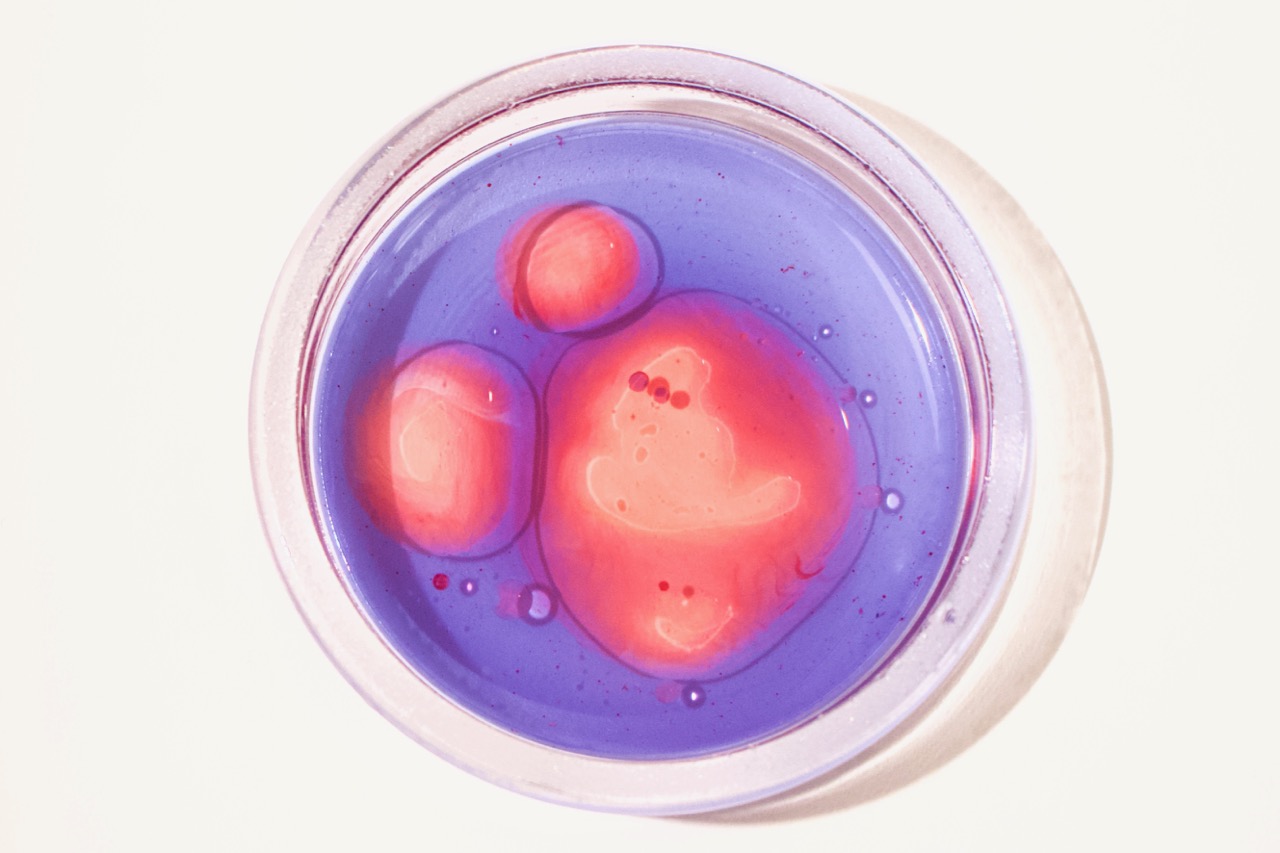
Take this one step further to explore how these dyes can help to better understand emulsions—mixtures of different substances that wouldn’t usually interact!
Materials

Corner Store Items
- Oil
- Food Coloring
- (optional) Alcohol
- (optional) Dish Soap
- (optional) Food emulsions (e.g. milk, mayonnaise, etc.)

Everyday Items
- Water
Lab Items
- Microcentrifuge Tubes, Test Tubes, or Petri Dishes (or substitute any small containers)
- Oil Red-O
- Ethanol
Preparation
Prepare stock solutions:
Oil Red O: 3 mg/ml in ethanol, mix well to combine.
Food coloring: add drops into water to desired brightness (e.g. 10 drops in 5 ml).
Procedure
- Label microcentrifuge tubes/test tubes/etc. Prepare each sample in duplicate (one for oil red-O and one for food coloring). Suggested conditions include: oil, water, oil/water.
- Add 10 drops of each sample to each tube.
- Oil: 10 drops of oil
- Water: 10 drops of water
- Oil/Water: 10 drops of oil + 10 drops of water
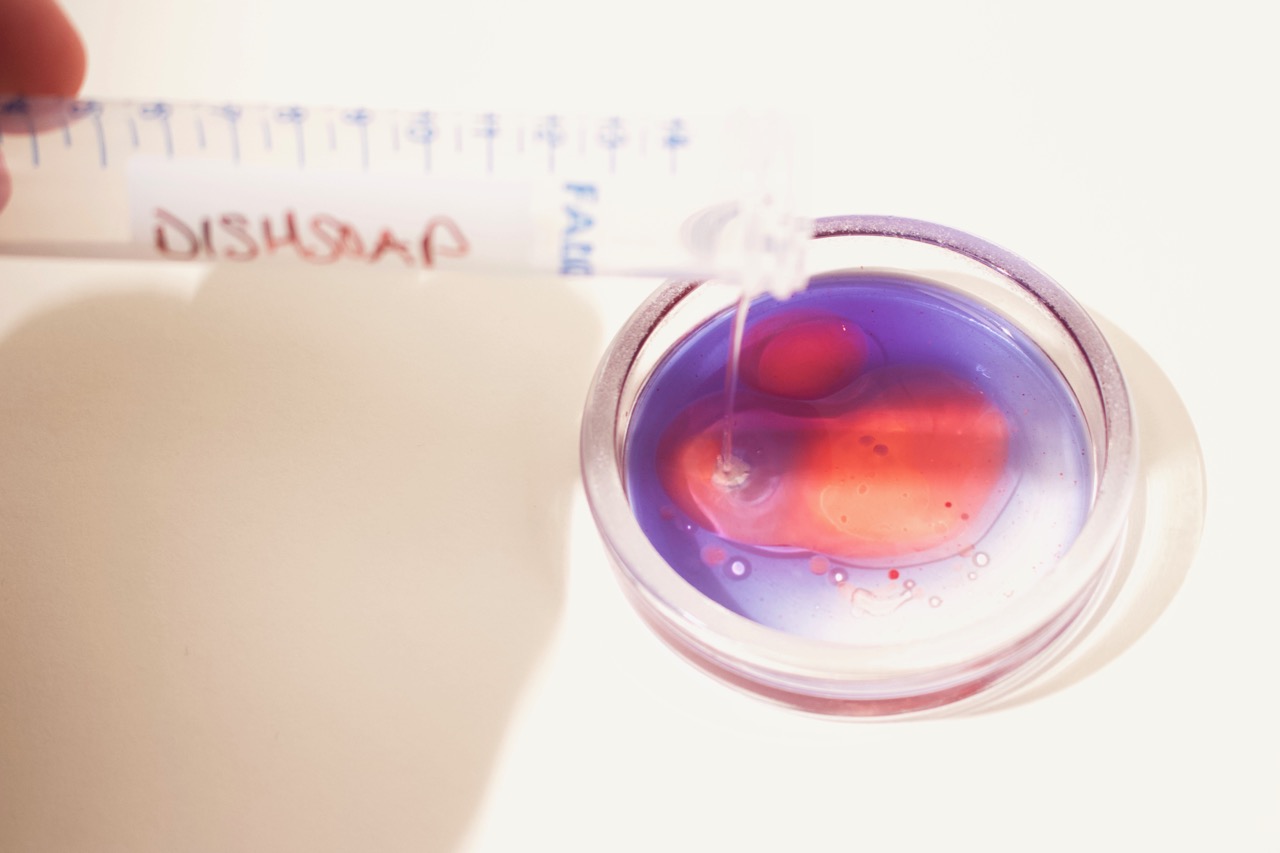
- Add one drop of food coloring or two drops of oil red-O to each tube.
- Shake or flick to mix. Then let sit to stabilize.
- Observe.
- Prepare additional samples with additives that can affect the interaction of oil and water (e.g. dishsoap, alcohol, etc.) or with emulsions (e.g. milk, mayonnaise, etc.).
Discussion
Describe the intermolecular interactions of the various molecules (oil, water, food coloring, Oil Red O, etc.)
Why was Oil Red O prepared in ethanol and not in water? If the powder was mixed with water, what might you expect to happen?
How can these dyes help us to learn about the chemical compositions of foods like mayonnaise? Or other applications?