What to know about soil?
Soil is more than just dirt—its complex microgeography consists of a wide variety of interdependent living and nonliving components. The nonliving can be separated into organic (carbon-based) and mineral matter, each having different physical and chemical properties, but each serving vital roles in the soil’s proper functioning. The living encompasses micro- and macro- organisms which contribute to soil structure and unlock vital nutrients for plant consumption. The absence of matter, that is, empty space, is also a vital component of soil as it allows for water storage and root permeation. Therefore, the health of soil is dependent on a balance between soil chemistry, physics, and biology. Understanding this balance is key to understanding soil health.
Soil is important to our lives—our access to nutritious food, clean water and air, fuel, medicines, and protection from drought and flooding are all dependent on healthy soil. Soil may even be a key for tackling climate change. Thus, maintaining healthy soil is essential to maintain our way of life. So…
What makes soil healthy?
And what can we do to promote soil health?
Let’s dive into the science behind this crucial resource!
What do you know?
Soil Chemistry
Nutrients
There are 17 essential nutrients for a plant’s growth. Three of these are derived from air and water, and account for 94% of a plant’s weight. These are carbon, oxygen, and hydrogen. The other 14 nutrients must come from the soil.
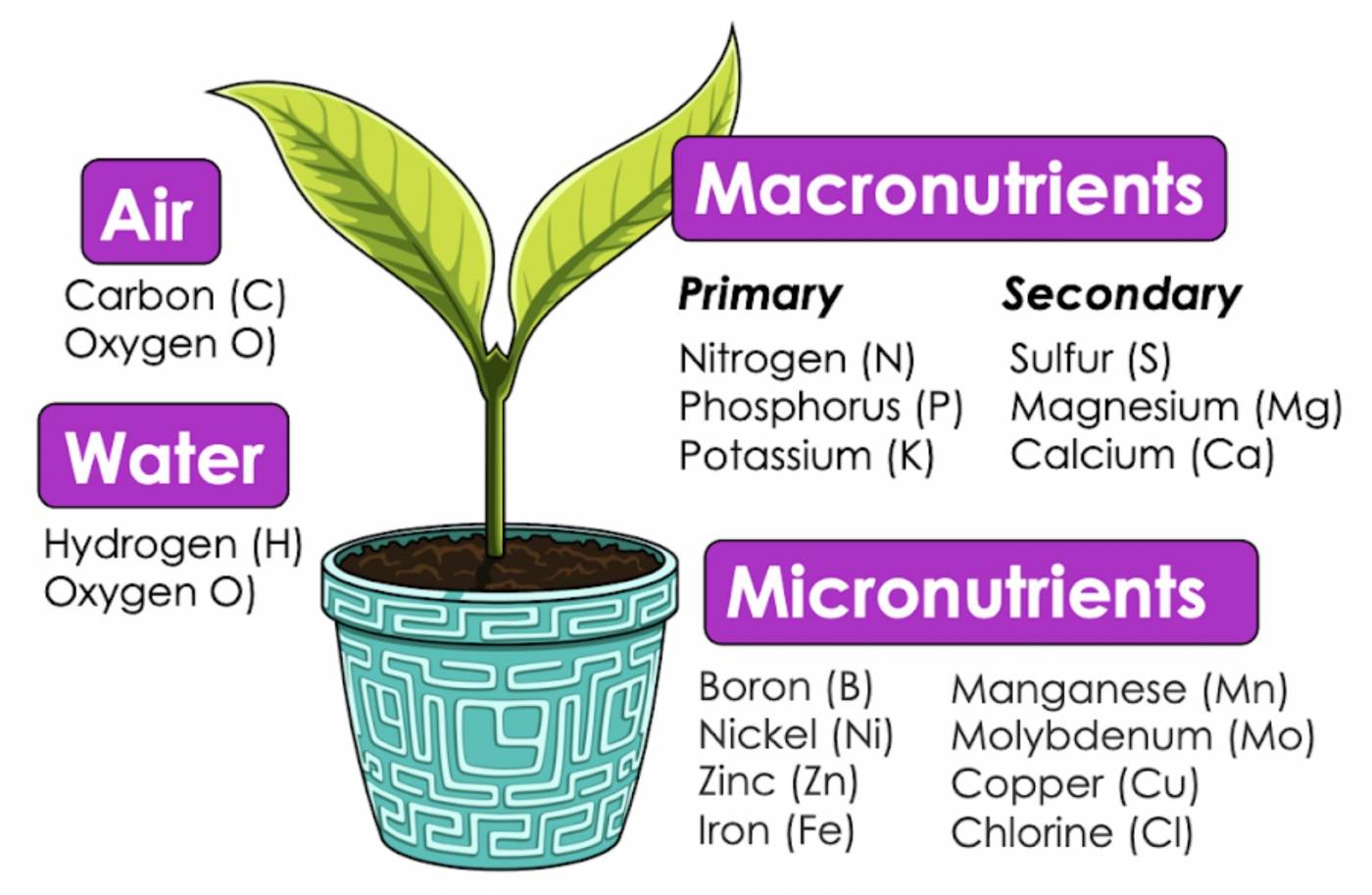
Figure 1: The 17 essential nutrients for plants. Macronutrients are consumed in extensive amounts, with primary macronutrients being the most heavily consumed. Micronutrients are required in trace amounts.
Nutrients in soil are divided into macronutrients (needed in larger quantities) and micronutrients (needed in smaller quantities). The figure below shows the elements that are classified as macro- vs micro- nutrients.
Roots can only absorb nutrients dissolved in water. The nutrients that are not dissolved in water can be found in solid form (newly applied fertilizer granules), in adsorbed form (bound to soil particles like clay and organic matter, Fig. 4), or in chemical compounds (decomposition products released by microorganisms).
If roots can only absorb dissolved nutrients, how does the plant make use of those locked away? Well, the soluble and insoluble forms of nutrients are in equilibrium with each other.
Think of the soil as a bank for plants. The dissolved nutrient pool is like a checking account and the undissolved pool is like a savings account. In the checking account, funds are immediately available. If the checking balance is low, transfers are made from the savings account to the checking account. Oppositely, if the checking account becomes full of money, some can be transferred to savings for storage. In the same way, when there is an excess of nutrients in the soil water, some can bind to soil particles and some can react to form insoluble minerals for storage. Here, they remain temporarily unavailable until they are needed again, at which point they can desorb or redissolve into the soil water.
Many of the nutrients in soil are in a cationic form, or positively charged. Electrostatics has a lot to do with the way these nutrients “stick” to the soil minerals. Later we will discuss why different types of soil have differing abilities to retain nutrients.
Soil Physics
Soil Composition
The physical landscape of soil is a mixture of solids, liquids, and gas. About 50% of soil volume is solid, and the other 50% consists of pockets of air called pores that are either empty, filled with water, or filled with living organisms.
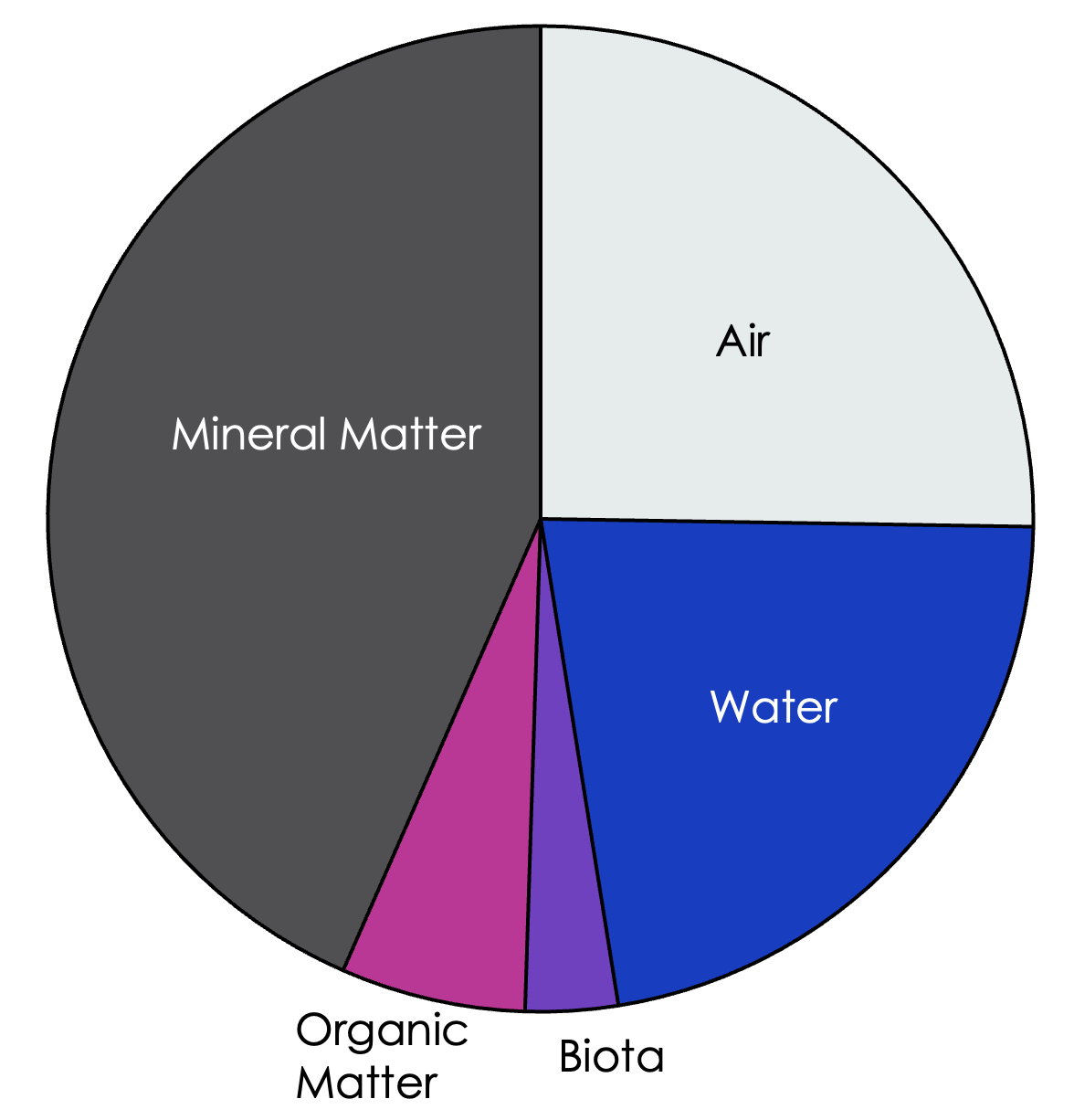
Figure 1: An example of the relative proportions of the components within a soil mixture.
The nonliving solids that make up soil consist of mineral matter (inorganic elements or compounds), and organic matter (carbon-containing compounds).
The proportions of water, pores, mineral & organic matter, and biota shown in the pie chart are a rough estimate of the general makeup of soil; these percentages vary from soil to soil and affect soil function. Here, we will discuss some of the most common physical parameters of healthy, productive soils – texture, structure, and porosity.