Review of Bonding and Intermolecular Forces
Bonds are sustained chemical connections between atoms. They hold sets of atoms together in predictable proportions or even precise numbers in order to create a myriad substances with diverse physical and chemical properties. The strongest bonds—covalent bonds—form discrete molecules that interact with one another through weaker intermolecular forces. It is this interplay between bonding and intermolecular forces that can be particularly challenging for high school students to grasp, and will be explored in-depth below.
Metallic Substances: Ductile, malleable solids
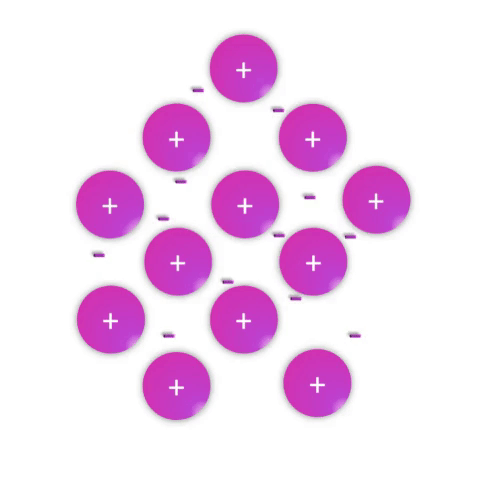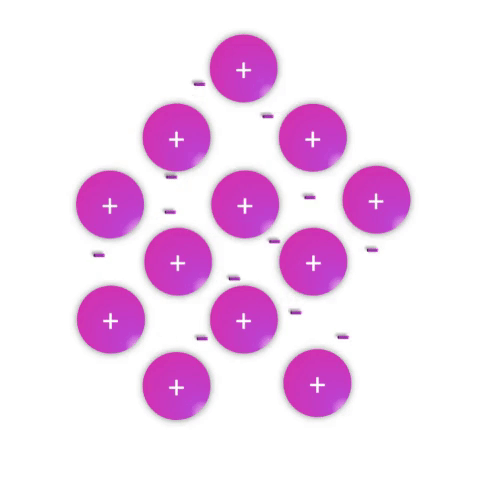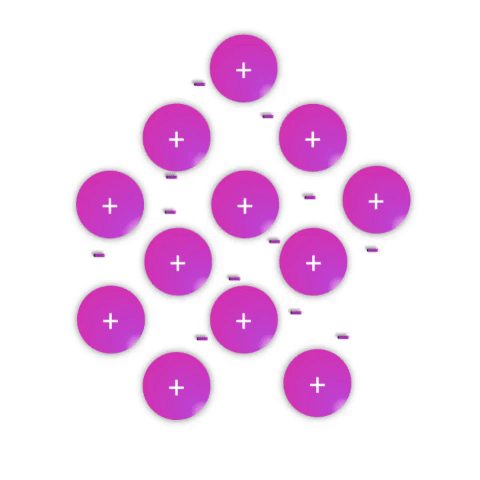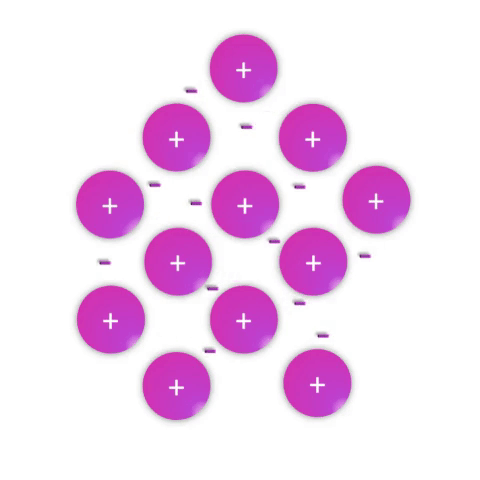 Metals—substances held together with metallic bonds—have properties familiar to most people as being solid at room temperature (with the notable exception of mercury!), conducting electricity, and often with the ability to be shaped (malleable) or pulled into wires (ductile). These properties demonstrate how the metallic bonds themselves are reasonably strong but subject to rearrangement with some force.
Metals—substances held together with metallic bonds—have properties familiar to most people as being solid at room temperature (with the notable exception of mercury!), conducting electricity, and often with the ability to be shaped (malleable) or pulled into wires (ductile). These properties demonstrate how the metallic bonds themselves are reasonably strong but subject to rearrangement with some force.
Metallic bonds are poorly understood and often explained as simply a “sea of electrons.” While this is definitely an oversimplification, it effectively conveys a connection between the atomic nature (metal atoms with few valence electrons weakly held by their nuclei) and the bulk properties. Metal atoms have affinity for one another sufficient to create a solid structure at room temperature, but the mobile electrons allow for conductivity and rearrangement of the atoms in space.
Ionic Substances: Crystalline, often water-soluble solids
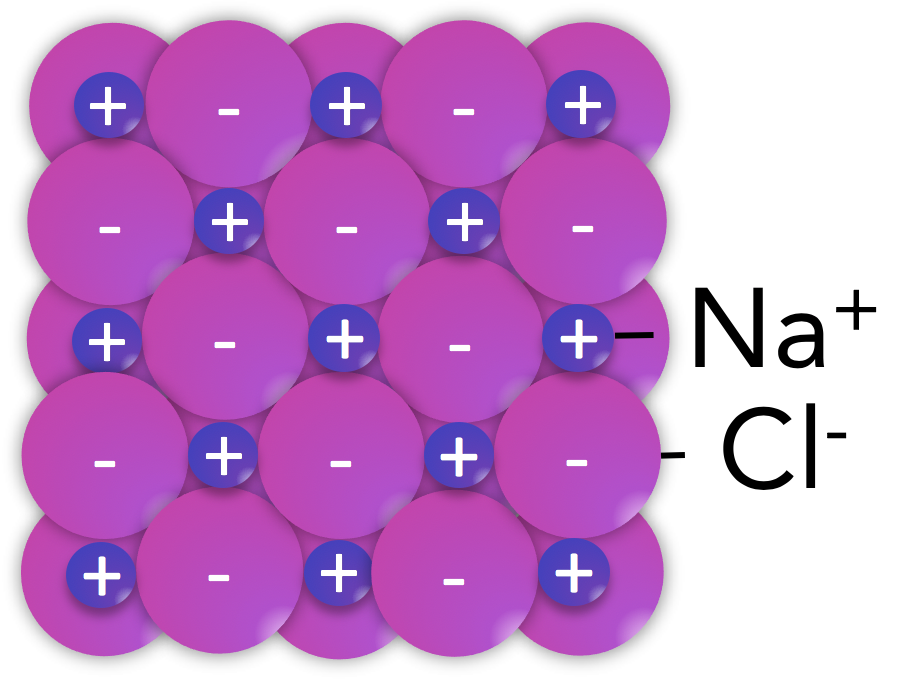 Ionic solids, or salts, are familiar as crystalline solids at room temperature that may break along smooth surfaces and often dissolve in water. Their crystalline nature, including smooth surfaces and predictable geometries, reflects the tight packing of ions into a rigid crystal lattice.
Ionic solids, or salts, are familiar as crystalline solids at room temperature that may break along smooth surfaces and often dissolve in water. Their crystalline nature, including smooth surfaces and predictable geometries, reflects the tight packing of ions into a rigid crystal lattice.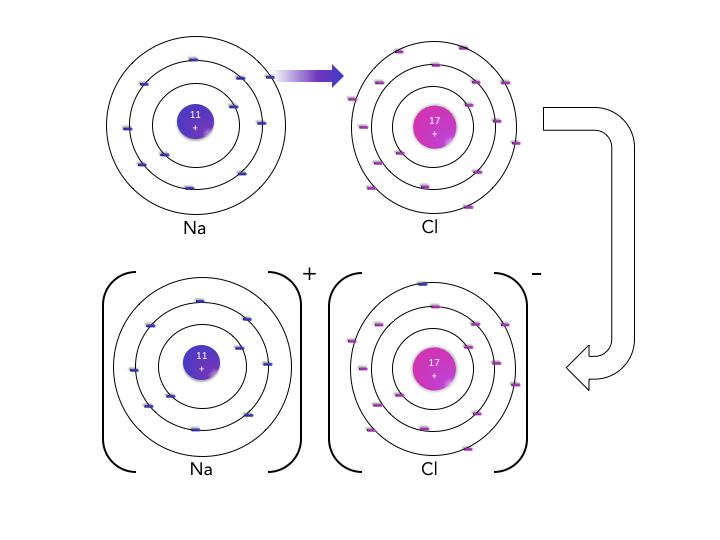
As the name implies, ionic substances are comprised of ions: positively charged cations formed from metal atoms that lost valence electrons and negatively charged anions from nonmetal atoms that gained valence electrons. These ions attract one another through electrostatic interactions that are distance dependent, so it is desirable for the cations and anions to be close together. These interactions do not depend on the geometry, or direction in space, so one positive ion attracts neighboring negative ions in all three dimensions.
Covalent Substances
Covalent Substances form a diverse class of substances that vary widely in their physical properties. This variation leads to student confusion about covalent bonds, but can also be resolved through careful examination of the relationships between covalent bonds and intermolecular forces, as explored more in the following pages.
 Covalent bonds are best introduced through examination of network solids, such as the covalent carbon network comprising diamond. Intermolecular forces can then be added into the picture by examining different classes of molecules, still with a variety of physical properties (such as carbon in graphite form). The range of intermolecular forces explain the variety of additional features observed.
Covalent bonds are best introduced through examination of network solids, such as the covalent carbon network comprising diamond. Intermolecular forces can then be added into the picture by examining different classes of molecules, still with a variety of physical properties (such as carbon in graphite form). The range of intermolecular forces explain the variety of additional features observed.
Covalent Substances, Part I — Network Solids: Solid, hard, and strong
Network solids form one class of covalent substances that are solids at room temperature and often known for their strength and/or hardness. Diamond is a particularly salient example of these properties, consisting of only repeating nonpolar covalent bonds between carbon atoms in all three dimensions.
Covalent bonds form through the sharing of electrons between two elements. As any one bond always arises through a pairwise interaction between two atoms, there is a limit to the number and spacing of these interactions based on the total pairs of shared electrons. Unlike ionic substances that have atoms packed as tightly as space will allow, most covalent substances are limited by the number of atoms involved in the sharing of electrons around the central atom. For example, this makes diamond significantly less dense than a salt crystal or metal. However, the shared pairs of electrons are very strong, stable interactions between atoms that lead to the strength and stability of network solids.
Covalent Substances, Part II — Molecules: Solids, liquids or gases with a range of properties
Molecular solids can be solids, liquids, or gases at room temperature and can display a range of other physical properties depending on the nature of the molecules comprising the substance itself as well as the nature of the interactions between the molecules. The interactions between molecules are the intermolecular forces, just as the highways that run between states are called interstate highways.
But before we look at the interactions between them, what are these molecules themselves? Because covalent bonding involves the sharing of electrons between pairs of atoms, the interactions are specific and the number of atoms involved is defined. Where an ionic substance will have an outermost cation that can still attract other anions, a covalently bonded atom will contribute a limited number of electrons for covalent bonding and once those are formed it will not be able to form additional covalent bonds. The most salient example here is water: H2O. One water molecule consists of exactly one oxygen and two hydrogens—no more, no less, and even no multiples (e.g. never two oxygens and four hydrogens).
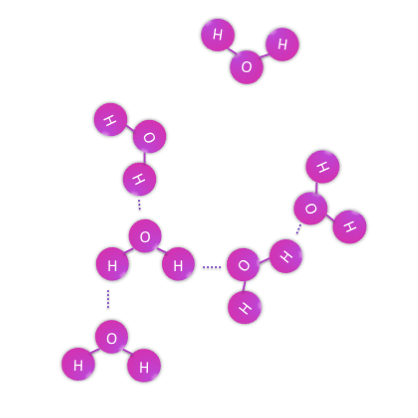 Continuing with the water example however, a glass of water does not contain one oxygen and two hydrogens, it contains many more and thus many water molecules. These molecules do not covalently bond with each other because they have already shared all of their possible pairs of electrons within each H2O, so they resort to weaker interactions between their surfaces, which we know as intermolecular forces. The properties of water, such as it being cohesive, adhesive, and liquid at room temperature all stem from the interactions between water molecules. When water changes phase, such as from a liquid to a gas, it is the interactions between molecules that change, while each H2O molecule remain essentially unchanged.
Continuing with the water example however, a glass of water does not contain one oxygen and two hydrogens, it contains many more and thus many water molecules. These molecules do not covalently bond with each other because they have already shared all of their possible pairs of electrons within each H2O, so they resort to weaker interactions between their surfaces, which we know as intermolecular forces. The properties of water, such as it being cohesive, adhesive, and liquid at room temperature all stem from the interactions between water molecules. When water changes phase, such as from a liquid to a gas, it is the interactions between molecules that change, while each H2O molecule remain essentially unchanged.
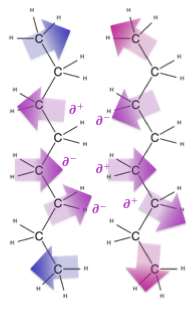
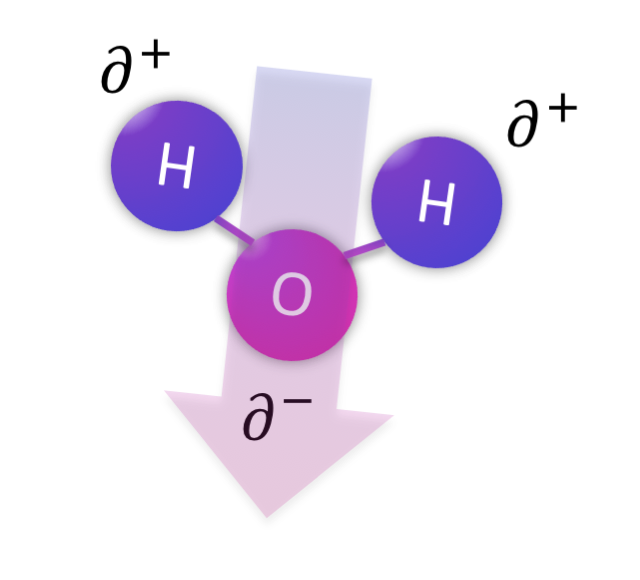 There are three main flavors of intermolecular forces depending on the properties of the molecule at hand. The most important distinction is whether or not the molecule is polar. If a molecule is polar, it has a separation of charge across the shape of the molecule, resulting in a part that is a bit positive (delta +: ∂+) and a part that is a bit negative (delta -: ∂-). The molecule overall has equal protons and neutrons so it is not an ion, it is just polar. Sometimes the separation of charge is called a dipole, with di- referring to the two types of electrostatic charge that are separated. Interactions between polar molecules are called dipole-dipole interactions.
There are three main flavors of intermolecular forces depending on the properties of the molecule at hand. The most important distinction is whether or not the molecule is polar. If a molecule is polar, it has a separation of charge across the shape of the molecule, resulting in a part that is a bit positive (delta +: ∂+) and a part that is a bit negative (delta -: ∂-). The molecule overall has equal protons and neutrons so it is not an ion, it is just polar. Sometimes the separation of charge is called a dipole, with di- referring to the two types of electrostatic charge that are separated. Interactions between polar molecules are called dipole-dipole interactions.
In some cases, molecules can form particularly strong dipoles and the interactions between these molecules can be particularly strong. This occurs when a very electronegative atom (N, O, F, and sometimes Cl) is bonded to a hydrogen atom (which only has one electron itself) to create a strong dipole that is attracted to a lone pair on a neighboring very electronegative atom (N, O, F, and sometimes Cl). This is known as a hydrogen bond, and water is a great example of covalent molecules held together by hydrogen bonds.
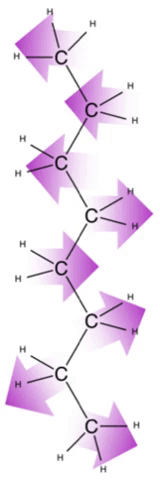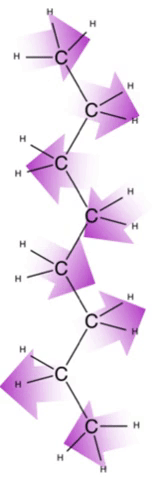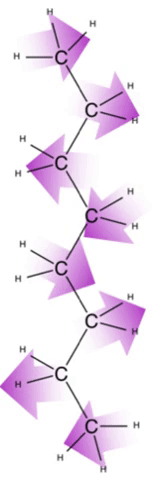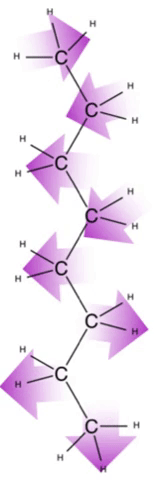 Finally, some molecules do not experience much of any separation of charge and are nonpolar. These molecules have weaker interactions with each other that are brief and random. Because the electrons are always moving around the nuclei of their atoms, there are periods of imbalance in charge between the negative electrons and positive nuclei.
Finally, some molecules do not experience much of any separation of charge and are nonpolar. These molecules have weaker interactions with each other that are brief and random. Because the electrons are always moving around the nuclei of their atoms, there are periods of imbalance in charge between the negative electrons and positive nuclei.
Such an imbalance can interact with a nearby molecule, causing them to be transiently attracted to one another. However, the electrons keep moving and this imbalance resolves itself so the interaction is not sustained. Each such interaction contributes to the dispersion forces felt between molecules. The larger the molecule, the more likely neighboring molecules will be to continue to have aligned imbalances and thus the stronger the overall effect of these interactions.