Which Carbs Do Yeast Like Best?
Background
All simple sugars have the same formula C6H12O6. Complex sugars and carbohydrates are chains of these sugars connected together (linearly, like beads on a string or branched, like a tree). The simplest of these is sucrose, or table sugar, which consists of two simple sugar units—one glucose and one fructose. Do the specific sugar structures matter? Does it matter whether individual sugars are bonded together or separate?
To understand how different sugar substrates are utilized by S. Cerevisiae (yeast), we can measure the amount of CO2 produced. If you recall the stoichiometry for fermentation, for every mole of glucose, yeast cells will produce two moles of CO2, which makes a quantification of sugar metabolism fairly straightforward. While scientists have invented a number of devices to quantifiably measure the rate CO2 production resulting from fermentation in yeast, these devices are not practical for classroom settings. Here we will use a basic 15ml conical tube (such as Falcon or Corning brands) with gradation markings as a device to measure CO2 production in response to a variety of carbon sources.
Materials

Foods
- 40% w/v: sugar and starch solutions e.g. glucose (corn sugar), fructose, sucrose (table sugar), starch, etc.
- Baker’s Yeast Packets (7% w/v in water)

Consumables
- 15mL Conical Tubes
- Needles
- Markers
- Pipette Tips
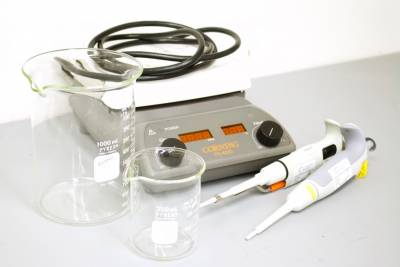
Equipment
- Water Bath or Hot Plate
- Beakers
- Timers
- Micropipettes
Preparation
- Prepare sugar solutions at 40% w/v (40g per 100ml of H2O)
- Heat water baths and/or hot plates to 40 °C
- Warm all solutions to be used
- Puncture 2-4 holes in caps of 15ml conical tubes using a 21g needle
- As CO2 is produced, it will displace the yeast-sugar solution via the holes punctured in the tube cap, and provide a volumetric measurement of gas produced over time.
- Immediately before experiment, prepare the 7% yeast solution in H2O
Setup
 Set up your tubes and water bath in such a way that the gas produced in each fermentation will rise to the tops of the tubes and volumes of gas can be marked with a permanent marker or waxed pencil.
Set up your tubes and water bath in such a way that the gas produced in each fermentation will rise to the tops of the tubes and volumes of gas can be marked with a permanent marker or waxed pencil.
In the example at right, plastic tubes were used with a few holes poked into each plastic cap. The tubes were filled with yeast solution and food source (e.g. glucose), covered, and inverted quickly into the water bath. The samples should be held inside the tubes and displaced into the water bath as gas is produced.
A similar setup can be used with test tubes and stoppers with holes or using a glass slide to invert each tube into the bath before sliding off the slide and allowing the fermentation gas produced to displace the yeast solution. You may also be able to use a typical gas collection apparatus from a chemistry laboratory at your school.
Finally, volumes of gas can be determined by emptying a marked tube and filling it with water exactly to the mark. Then that water can be poured into a graduated cylinder of appropriate size to determine the volume of gas produced.
Procedure
- Fill 15ml conical tube with 8ml of a sugar solution (recommended starting concentration is 0.5%, v/v)
- Fill remainder of tube (~7ml) with 7% yeast solution such that the meniscus rises above the lip of the tube.
- NOTE: stock yeast solution should be agitated before adding to tube
- Replace cap onto tube — because of holes, there will be a small squirt of solution to come out.
- NOTE: make sure that there are no sizeable air bubbles in the tube
- Invert the tube, and place in a large beaker filled with water (preheated to 40 °C). Place this beaker into a water bath or onto a hot plate to maintain temperature
- NOTE: you can also test the effect of temperature on fermentation by adjusting temperature of water bath or hot plate
- Immediately mark the bottom of the CO2 bubble (if there is one). Mark this point at 5 minute intervals for 30 minutes.
- At the end of the experiment, record the level of CO2 produced at each time interval
Which substrate(s) did the yeast ferment fastest? Which substrate(s) allowed for the greatest total fermentation?
Based on your results, what does this tell you about the important bonds in sugar and starch molecules?
Which of these food molecules are in your egg sandwich? How do you think your body compares to the yeast when trying to digest these sugars and starches?