Protein Denaturation and Digestion
Background
Everyday foods are an excellent way to introduce important concepts related to protein denaturation and digestion. For example, milk consists of a variety of nutrients, including about 3% proteins. Of these proteins, about 80% consist of the relatively hydrophobic casein proteins that exist in micelles and give milk its cloudiness, with the other approximately 20% of milk proteins consisting of a variety of water-soluble (whey) proteins.
 Casein proteins are heat-stable, but will denature below pH 4.6. This can be seen from the familiar milk curdling in acid (buttermilk, ricotta cheese, etc.). These proteins are held together by phosphate groups that bind to calcium ions, linking the casein chains together. When introducing an acid, the ionic interactions between the casein phosphate groups and calcium ions are disrupted, causing the casein proteins to denature. Interestingly, this chemistry is the basis of cheesemaking — caseins are also substrates of the protease rennet, which is added to milk in the early stages of making fresh cheese.
Casein proteins are heat-stable, but will denature below pH 4.6. This can be seen from the familiar milk curdling in acid (buttermilk, ricotta cheese, etc.). These proteins are held together by phosphate groups that bind to calcium ions, linking the casein chains together. When introducing an acid, the ionic interactions between the casein phosphate groups and calcium ions are disrupted, causing the casein proteins to denature. Interestingly, this chemistry is the basis of cheesemaking — caseins are also substrates of the protease rennet, which is added to milk in the early stages of making fresh cheese.
The water-soluble whey proteins are diverse, and many are rich in sulfur. These proteins can be denatured at high temperatures, leading to the formation of milk skin. Whey proteins are not as acid-sensitive as caseins, thus remain in the whey solution during acidification (such as for cheese making). These proteins are still susceptible to other proteases, such as those introduced by bacteria when making yogurt.
 An accessible source of protease is meat tenderizer, which includes papain (from papaya) or bromelain (from pineapple). These enzymes will cleave caseins over time, and contribute to the coagulation of milk. As such, we are using meat tenderizer as the basis for this experiment.
An accessible source of protease is meat tenderizer, which includes papain (from papaya) or bromelain (from pineapple). These enzymes will cleave caseins over time, and contribute to the coagulation of milk. As such, we are using meat tenderizer as the basis for this experiment.
To take this one step further, look into getting some rennet, an enzyme very commonly used in cheese-making. It can be used in this experiment in similar ways to meat tenderizer.
Materials

Foods
- Unseasoned Meat Tenderizer
- Vinegar
- Milk

Lab Supplies
- Test Tubes or small beakers
- Pipettes or Measuring Spoons
- Hot plate
Preparation
Track down unseasoned meat tenderizer, which can be hard to come by in the store. Avoid the seasoned version as the color of the seasoning makes it more difficult to observe your results. You may have to order it online — it is available on Amazon if you can’t find it in your local grocery store.
Note
To make this most relevant to an egg sandwich, consider also getting some rennet, and using it in similar ways to the meat tenderizer in this experiment. This can even be a great inquiry step for students to design their own conditions for testing the effects of rennet and providing the necessary controls.
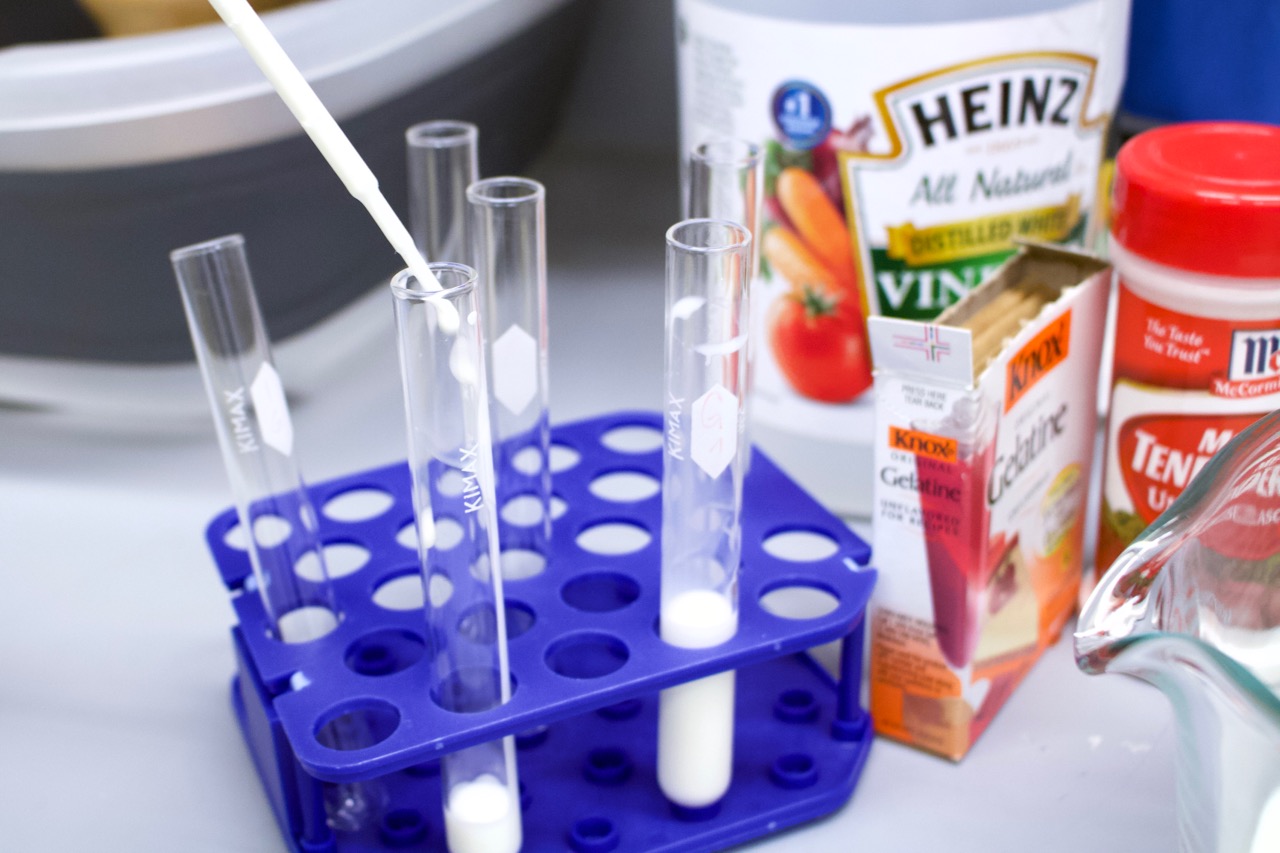
Procedure
PROCEDURE FOR MILK TEST
- Fill 6 test tubes each with 5 mL of milk.
- To each test tube containing milk, add one of the following substances:
- 1 mL water
- 1 mL vinegar
- 2 g meat tenderizer
- 1 mL meat tenderizer solution
- 1 mL boiled meat tenderizer solution
- 1 mL boiled vinegar
- Make observations immediately and over the next 30-60 minutes.
What conclusions can you draw about how acid, heat, and enzymes affect milk protein structure?
Which conditions might be starting to make “cheese?”
Discussion
Explain the function of water in the experimental design set-up.
How do the boiled additives compare to the original samples? Why were some affected by heat while others were not?
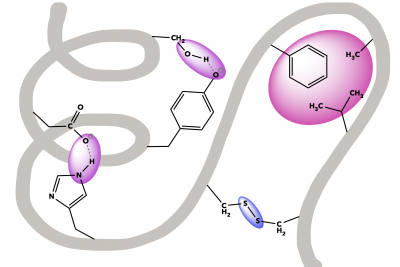
Describe the difference between denaturation and digestion of proteins. Draw a diagram to fully explain your answer (or modify the one at right).
Use your model to propose what you think might be happening when cheese is made.