Characteristics of an Emulsion
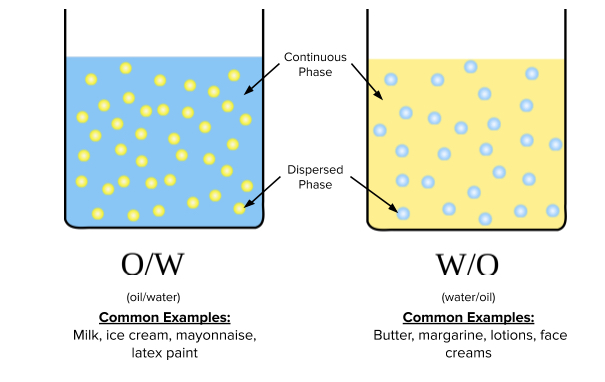
As mentioned above, an emulsion is a type of colloidal mixture where normally immiscible liquids are combined in a way that maintains their unique chemical identities. In general, there are two parts of an emulsion:
Continuous Phase: the liquid portion of an emulsion in which another liquid is dispersed
Dispersed Phase: the liquid portion that forms tiny droplets that are evenly suspended throughout the continuous phase
The schematic below represents two configurations of oil and water emulsions. On the left, an aqueous solution (water-based) is the continuous phase in which droplets of oil are dispersed. Conversely, the right side depicts oil as the continuous phase, with droplets of water dispersed throughout. Again, it is important to note that the components of the continuous phase do not interact with the dispersed phase — nothing is dissolved.
Upon zooming in, we can see how a surfactant can help stabilize an emulsion
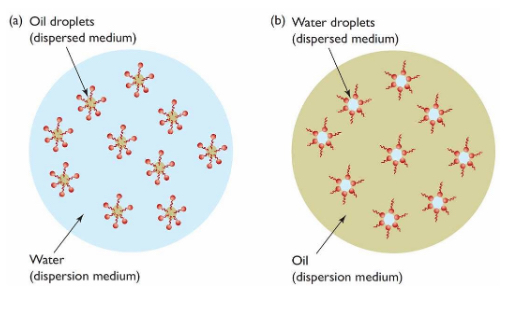
by forming micelle “cages” around dispersed droplets. Because of the amphipathic nature of surfactants, the dispersed droplets can be protected from the continuous medium in both scenarios depicted above.