History and Biochemistry of Fermented Foods
While the process of fermentation significantly predates the emergence of our species, humans have been harnessing this microbial technology for food production and preservation for thousands of years. In many cases, specific fermented foods can act as a marker of cultural identity, and perhaps draw us back to the flavors and smells of grandma’s kitchen. Here, we will dive into the history and science of fermented foods.
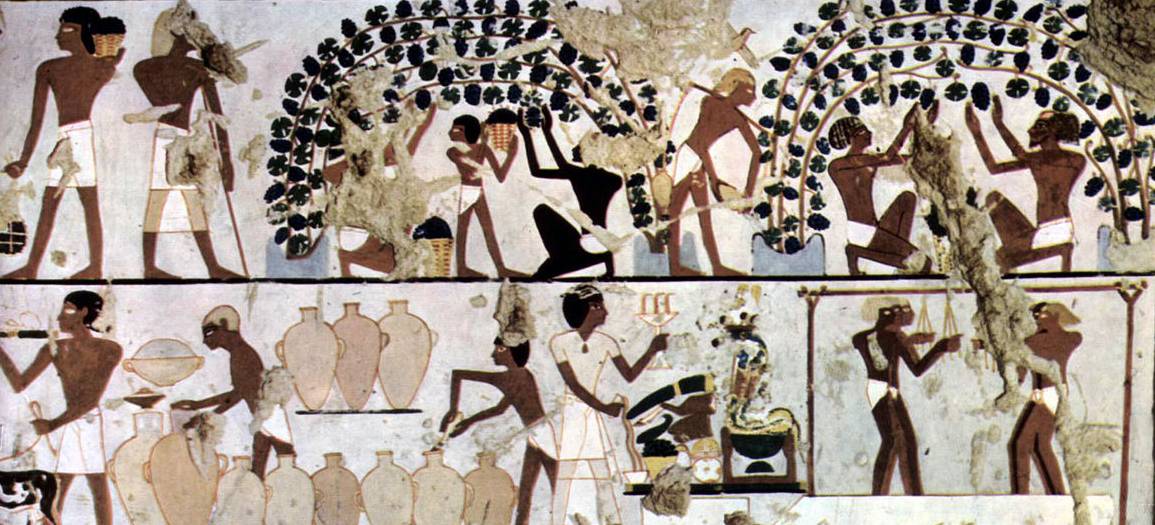
A portion of an ancient Egyptian artifact depicting grape cultivation and winemaking. Source: Ägyptischer Maler um 1500 v. Chr. [Public domain], via Wikimedia Commons
A Brief History
In the culinary sense, fermentation is the transformation and preservation of food by bacteria. While the process of fermentation as a culinary practice dates back to early human civilization, it took a very long time before the scientific principles were understood (1). However, archaeological studies have shown that fermentation technologies were well-established components of ancient civilizations, and there is even evidence that the concept of “starter” cultures were widely appreciated and maintained (2-5). The table below highlights a few fermentation milestones in the context of culinary anthropology:
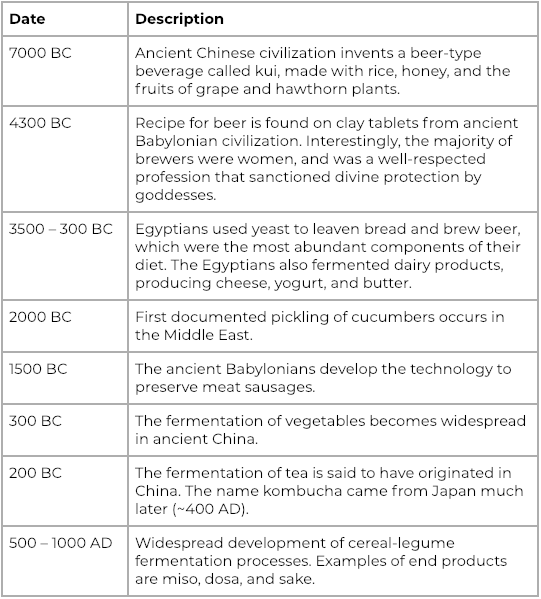
Selected fermentation milestones over human history. Table adapted from “The History of Fermented Foods.” (2)
The Dawn of Biochemistry

A not-so-young Louis Pasteur
Despite the incredible and rich anthropological history surrounding the use of fermentation for food preservation, it was not until more “recent” times when we started to learn that fermentation was a metabolic process inherent to microorganisms. As the seeds of microbiology were sown through the discovery of bacteria by Antonie Van Leeuwenhoek (1683), the modern scientific enterprise began to take shape, and fermentation was at the center of this movement. At first, it was presumed that the decay and death of microorganisms gave rise to fermented foods, as theorized by German chemist Justus Von Liebig (6). However, in 1857, a young French scientist by the name of Louis Pasteur made an interesting observation that challenged this notion — and it was all because of a problem with the industrial production of an alcoholic beverage (7).
The juice for beets is a historically fermented beverage
A year before Pasteur published these important observations, he was approached by an industrialist from Lille, Monsieur Bigo, who made alcohol from beet juice. Many vats of Bigo’s beet juice were not being converted into alcohol — instead, the juice was souring (more like vinegar). This phenomenon threatened his business, so Pasteur came to investigate.
Pasteur, in part, used microscopy to understand the chemical and biological basis of fermented foods
Using a microscope, Pasteur looked at samples from the successful vats (containing alcohol) and noticed the presence of nice, fat globules. However, in looking at the soured samples, he only saw thin, elongated particles. Through further study, he also concluded that the soured sample contained acetic acid, which is definitely not alcohol!
To help make sense of this difference, he went on to characterize the alcohol produced as the beet juice was “successfully” fermented, and Pasteur found this compound to be optically active. He had already been studying stereochemistry for several years, and his previous observations suggested that all organic compounds with optical activity were formed by living organisms (8). Thus, he came to the conclusion that live cells were responsible for the metabolic process of fermentation:
The chemical changes of fermentation are associated with a vital activity, beginning and ending with the latter. I believe that alcoholic fermentation never occurs without either the simultaneous organization, development and multiplication of cells or the continued life of cells already formed. All the results in this paper seem to me completely in opposition to the opinions of Liebig and Berzelius…Now…in what does the chemical act of decomposing the sugar consist; and what is its precise cause? I confess that I simply do not know. — Louis Pasteur (8)
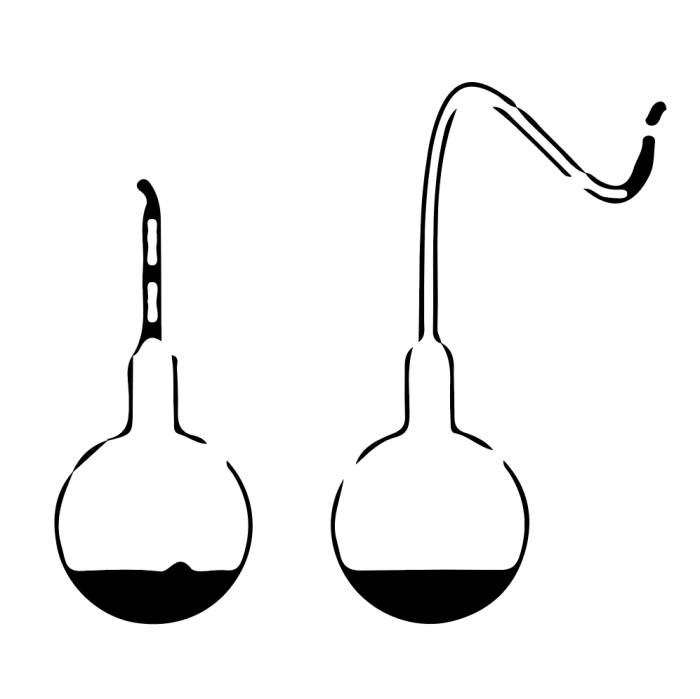
Pasteur used swan-necked flasks (right) to demonstrate that living particles from the air were a source of contamination—rather than the previously held theory of spontaneous generation
Pasteur continued to study fermentation and discovered that yeast convert sugars into alcohol, and that the “souring” effect of the beet juice was the result of contamination with a bacterial species that is capable of converting ethanol into acetic acid. To eliminate possible bacterial contaminants, Pasteur pioneered a technique where samples were heated to a specific temperature for a specific length of time. This process, known as pasteurization, is still widely used today.
While there was still much to learn, the major tenets of Pasteur’s work on fermentation were groundbreaking. Building on this scientific story was Moritz Traube, a German chemist who in 1877 proposed that fermentation was a cascade of chemical events where oxygen is transferred from one part of a sugar molecule to another, resulting in a highly oxidized product (CO2) and a highly reduced product (i.e. alcohol). Furthermore, Traube suggested that each chemical event in the fermentation sequence is catalyzed by a protein-like substance (alluding to enzymes), providing remarkable insights into cellular chemistry well before others in the field (1). Twenty years later, Eduard Buchner, also a German chemist, demonstrated that sucrose could be fermented to alcohol by yeast extracts, coining the term “zymase” to describe the cellular compound that catalyzes this conversion. Buchner’s work was worthy of the 1907 Nobel Prize in Chemistry, and he modified Pasteur’s model to stress the idea that fermentation is a function of living, but not necessarily dividing, cells. Furthermore, Buchner showed that fermentation is a chain of events, with each step catalyzed by a different enzyme (9).
Through their work on the scientific process of fermentation, Pasteur, Traube, Buchner, and many others, paved the way for the study of the chemistry of life, otherwise known as biochemistry, breaking open an entire scientific field.
Fermentation: La vie sans l’air
From a biochemical point of view, fermentation is a metabolic process through which organic compounds are converted into energy, without the involvement of an oxidizing agent. As Louis Pasteur succinctly suggested, fermentation is “la vie sans l’air,” or “life without air.” However, fermentation is not one size fits all. There is actually incredible diversity when it comes to fermentation processes, as different microorganisms house different mechanisms for the conversion of glucose into energy.
At the core of cellular respiration, which can include fermentation, is the highly conserved process of glycolysis. Through this ten step reaction, a molecule of glucose is split into two molecules of pyruvate. Glycolysis does not require oxygen, and is found in every living organism on earth, suggesting it was one of the first biochemical pathways to evolve. The fate of pyruvate, however, is dictated by the presence or absence of oxygen. When oxygen, an electron acceptor, is present, the pyruvate will shuttle into aerobic cellular respiration pathways — the Krebs Cycle and Electron Transport Chain — to generate ATP. In the absence of oxygen, however, pyruvate molecules will enter the fermentation cascade.
Some microorganisms have evolved a level of metabolic flexibility, allowing them to switch between aerobic respiration and fermentation. As such, these microorganisms are classified as facultative anaerobes. However, other microorganisms are poisoned by oxygen, and can only undergo anaerobic respiration. In this case, the microorganisms are obligate anaerobes. For both facultative anaerobes and obligate anaerobes, the process of fermentation serves to recycle the products of glycolytic reaction, ensuring that cells can continue producing ATP.
Consider the formula for glycolysis:
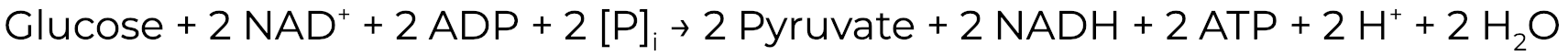
When oxygen is not present, glycolysis is the primary energy producing mechanism, generating 2 net molecules of ATP per molecule of glucose. In addition to having access to a sugar, glycolysis requires the coenzyme nicotinamide adenine dinucleotide (NAD). Specifically, the glycolytic enzyme glyceraldehyde phosphate dehydrogenase (GAPDH) requires NAD+ as a cofactor, which gets reduced to NADH during the enzymatic reaction. However, cells do not have an unlimited supply of NAD+, which poses a problem for producing energy. Therefore, it is really important for cells to have a mechanisms that oxidizes NADH back to NAD+. In many organisms, this is accomplished by fermentation.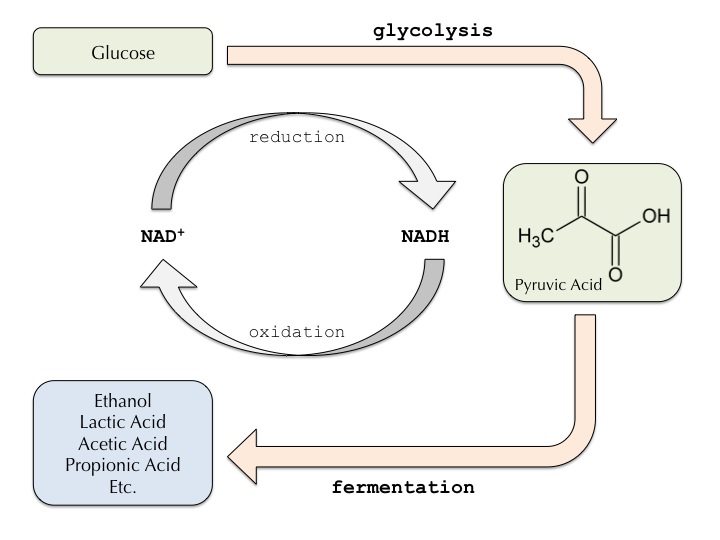
The applications of fermentation chemistry are many and varied, and extend beyond the kitchen. However, for the purposes of this discussion, we will focus on the fermentation reactions harnessed for culinary functions, namely the production of lactic acid and ethanol.
Lactic Acid Fermentation
The conversion of pyruvate to lactic acid occurs directly via the enzymatic action of lactate dehydrogenase. In this reaction, pyruvate is reduced to lactic acid, while NAD+ is regenerated and shuttled back into the glycolytic pathway. The Lactobacillales order, also known as Lactic Acid Bacteria (LAB), is a collection of gram positive bacteria that produce lactic acid at the end of carbohydrate fermentation cascades. Many LAB species are harnessed for food fermentation since the acidification of the growth environment (milk, brine, etc) prevents the growth of other microorganisms that can “spoil” food. As such, lactic acid fermentation has been used by countless cultures for centuries for food preservation. More recently, LAB bacteria have been shown to be a ubiquitous part of a healthy microflora profile in the human intestinal tract, contributing to their notoriety. Common foods containing LAB are yogurt, kimchi, sauerkraut, and lambic beer.
Ethanol Fermentation
The transformation of pyruvate to ethanol and carbon dioxide occurs over two steps. First, the carboxyl group is removed from pyruvate by pyruvate decarboxylase to yield acetaldehyde and carbon dioxide (this step is why alcoholic beverages can be carbonated). Then, the acetaldehyde is converted into ethanol through the action of an alcohol dehydrogenase, which produces NAD+ in parallel — providing a necessary substrate for glycolysis to continue. Yeast are very important facultative anaerobes as they are very effective in producing ethanol, allowing humans to “catch a buzz” when drinking beverages like wine and beer. Furthermore, this process is essential for breadmaking. While the majority of ethanol is cooked out of bread, the release of CO2 during the ethanol fermentation process is what makes loaves fluffy. 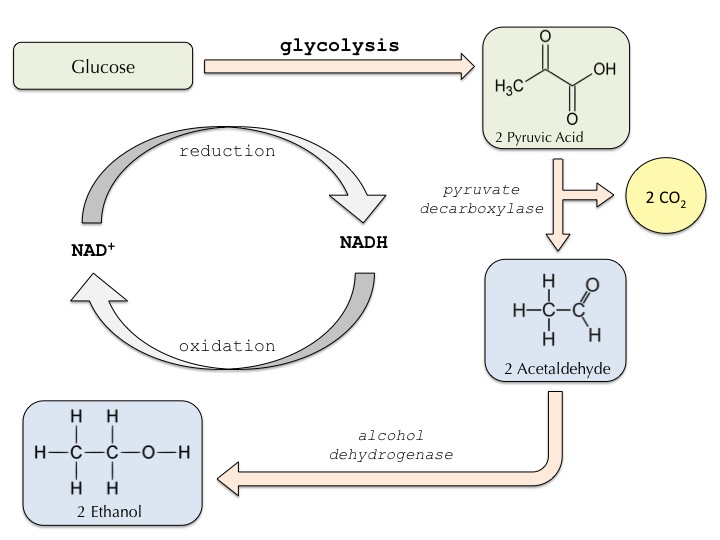
From a culinary standpoint, this creates an opportunity to create a vast array of food products and flavor profiles. Many of us have enjoyed the fruits of microorganism labor, and it is estimated that as much as one third of all food consumed by humans on planet earth is fermented (10). Pickles, olives, bread, beer, wine, chocolate, coffee, vinegar, soy sauce… All of these delicious foods, as well as many, many other types of ferments, are the result of shuttling pyruvate into some fermentation cascade. Sometimes kitchen fermenters utilize a “wild fermentation” process in which they harness the natural collection of microorganisms present on foods to create a specific culinary result. Other times, a well-defined and characterized starter culture is used to create specific fermented food products. It should be mentioned that fermentation is useful in industrial settings, too, and many biotechnology companies are pouring incredible resources into the research and development of industrial fermentation (i.e. biofuel).
Regardless of strategy, it is important to remember that microorganisms in nature do not exist in monoculture — meaning, in any given ecological setting, there are a variety of microorganism species living and working together in response to their environmental conditions.
Bibliography
- Fermentation Microbiology and Biotechnology. Third Edition. 3, illustrated, revised ed. CRC Press; 2011.
- Prajapati JB, and Nair BM. The History of Fermented Foods. Handbook of Fermented Functional Foods, edited by Farnworth ER, 2nd ed., CRC Press, 2017, pp. 1–22.
- Wang J, Liu L, Ball T, Yu L, Li Y, Xing F. Revealing a 5,000-y-old beer recipe in China. Proc Natl Acad Sci U S A. 2016;113(23):6444-8. [PDF]
- Breidt F, McFeeters RF, Perez-Diaz I, Lee CH. Fermented Vegetables. Food Microbiology: Fundamentals and Frontiers, edited by Doyle MP and Buchanan RL, 4th ed., ASM Press, 2013, pp. 841-855. [PDF]
- Blandinob A, Al-Aseeria ME, Pandiellaa SS, Canterob D, Webba C. Cereal-based fermented foods and beverages. Food Research International. 36 (2003) 527–543. [PDF]
- Liebig on the Fermentation of Wine and Beer. Scientific American. 1852:34. [HTML]
- Barnett JA. Beginnings of microbiology and biochemistry: the contribution of yeast research. Microbiology. 2003;149(3):557-67. [PDF]
- Barnett JA. A history of research on yeasts 2: Louis Pasteur and his contemporaries, 1850–1880. Yeast. 2000;16(8):755–771. [PDF]
- Buchner E. Cell-free Fermentation. nobelprize.org; December 11, 1907. [PDF]
- Katz S. The Art of Fermentation: An In-Depth Exploration of Essential Concepts and Processes from around the World. Chelsea Green Publishing; 1St Edition; 2012.