Explore Emulsions
These activities will allow students to explore how emulsions form, what type of emulsions can be formed, and how they can be applied in our daily lives.
Explore this...
Protocol: Lotion Recipes w Art
Background
Here we are focusing on a well-known emulsion — lotions to explore intermolecular forces. In completing this protocol, you will create a lotion where water droplets are dispersed in a continuous phase of different oils. Of course, the ratios of oils and water in this protocol are just suggestions! Feel free to manipulate these ratios to see what kind of consistency and texture you get. Remember, there is no right or wrong!
Materials
Common items
- Writing utensils for labeling
- A notebook, binder, or digital document for record keeping

Head to the Kitchen, Grocery Store, or Online Store
- Water — you can use tap water or purchase distilled water
- Oils — we are using a mix of sunflower and grapeseed, but lots of oils work!
- Emulsifying Wax
- Stearic Acid
- Glycerin
- Essential oils for aroma (optional)
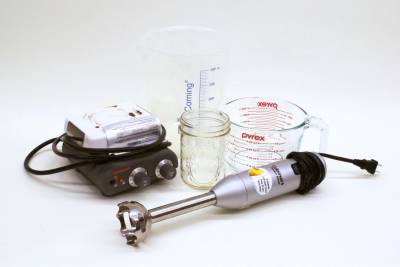
Equipment
- Containers for mixing — large bowls or beakers have been helpful in our experience
- Utensils for scraping — regular spoons work, but we love using rubber spatulas
- Kitchen Scale — set to grams
- Stick Blender, countertop blender, whisk, or other method/tool to provide a mechanical mixing force
- Kettle, saucepan, or microwave to heat water
- Double Boiler/water bath, saucepan, or microwave to warm oils and melt wax
- Containers, with lids, for storing lotion
Uncategorized
- A flat, uncluttered, and cleaned surface for making your lotion
- Whatever you need to clean as you go
Classify Simple Emulsions
Background
One way to classify emulsions is by identifying the continuous and dispersed phase of the emulsion. Continuous phase is the liquid in which another liquid is dispersed. In other words, it is the dominant phase in the emulsion. Dispersed phase is the opposite. It is the liquid that is dispersed within the continuous phase and which therefore occupies a relatively small space in the emulsion compared to the continuous phase.
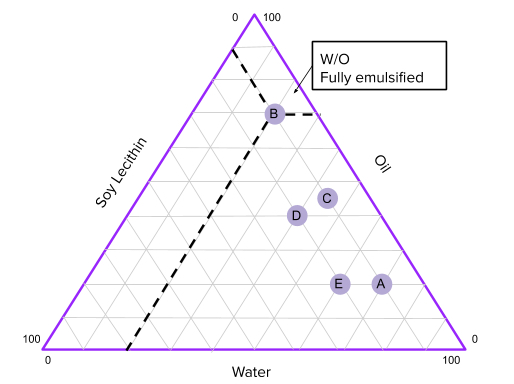
Based on this method of classification there are three major types of emulsions: O/W(Oil in Water), W/O(Water in Oil), and bicontinuous (where both water and oil have equal share in the emulsion). In this experiment, we will create different emulsions and observe what physical characteristics they exhibit and whether or not there is a more stable way to form an emulsion, that is, so that the emulsion stays emulsified for a long time without separating. After that, we will plot the characteristics on the phase diagram to estimate what ratios of water,oil, and surfactant will create the most stable emulsion.
Materials

Equipment
- Scale
- A stick to scrape off residue
- Mortar and pestle
- Vortex
- Rubber band
- Labels and pens
Consumables
- Capped plastic or glass tubes
- Weigh boats

Printables
Viscosity Test Paper (consistometer paper), emulsion observation table, and emulsion phase diagrams are provided in the download section on the right.
A Viscosity Test to Characterize Emulsions
Materials
Samples
- Emulsions that you want to test

Equipment
- Pipette stand/clear smooth surface
- consistometer paper (found in Downloads)
- pipette
- pen
- calculator