The Chemistry That Holds Us Together
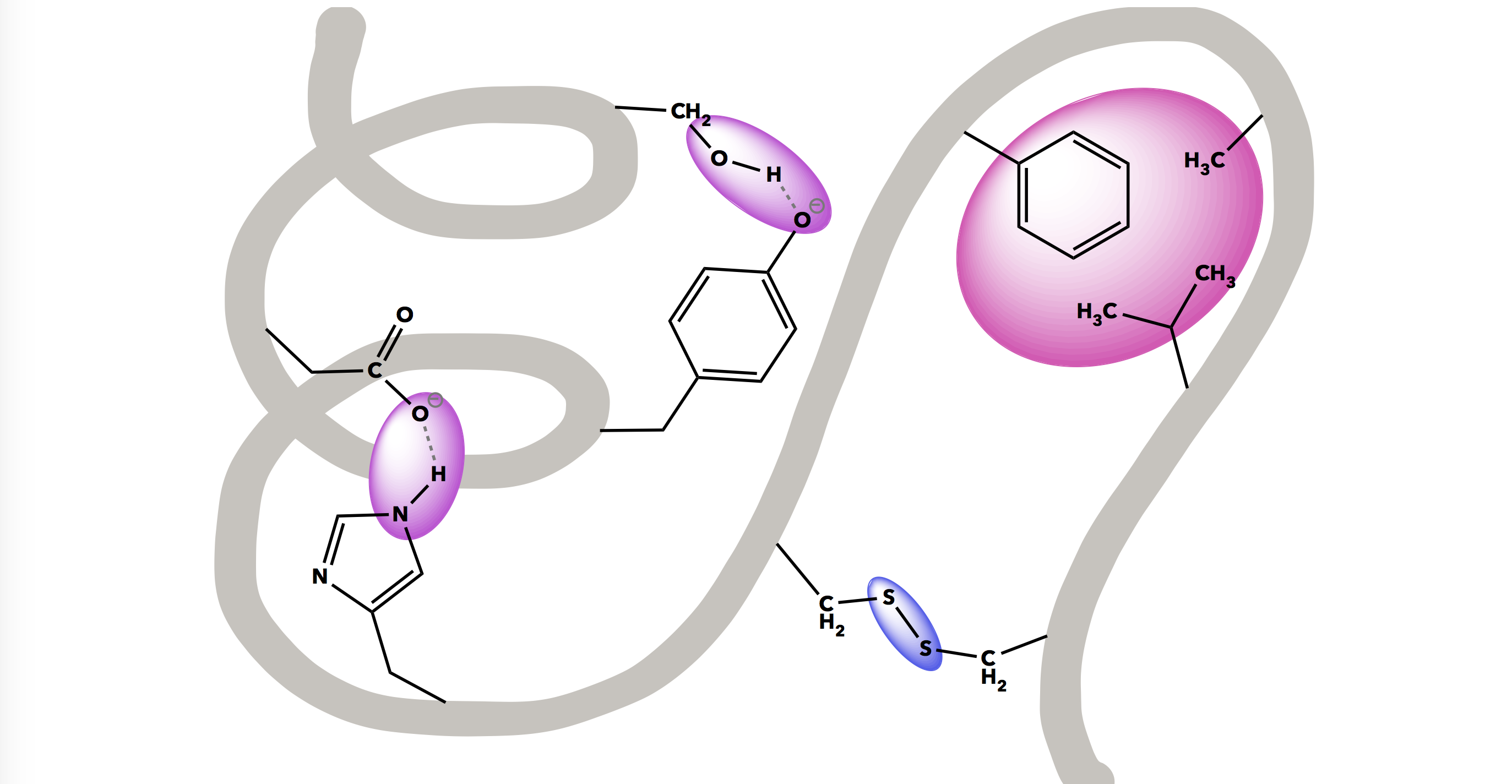
A human body is made of an estimated 100,000 unique molecules consisting of mostly hydrogen, oxygen, carbon, and nitrogen arranged in different configurations. Chemical bonds and interactions are central to this molecular diversity, and dictate the useful biological functions of molecules. It is this interplay between bonding and intermolecular forces that can be particularly challenging for students to grasp.
While biological macromolecules (proteins, lipids, carbohydrates, and DNA) are large and complex, they demonstrate various bonding and intermolecular interactions in clear, functionally-relevant ways. These macromolecules will be explored in-depth throughout this resource.
Choose a Level
At a glance
Topics
- Life Science,
- Chemistry of Life,
- Diversity of Organisms,
- Genetics,
- Physical Science,
- Bonding,
- Chemical Reactions
Materials Required
- Corner Store Materials,
- Lab Materials,
- Specialty Materials