Dyes and Pigments
Background
Different molecules absorb different wavelength of electromagnetic radiation, and reflect the remaining one, which is the color we see. For example, chlorophyll, a pigment found in many plant leaves absorbs blue and red light, reflecting green light back into our eyes, making plants appear green. Another illustration of absorption are sunglasses – they only let a small proportion of the electromagnetic radiation from the sun pass through the lenses into our eyes, protecting them from harmful UV radiation and intense light. In addition to absorption, most sunglasses also reflect light, which also keeps it out of our eyes.

Hydrangeas can change color from pink to blue depending on the pH of their soil
The structure of a molecule determines the wavelengths it absorbs. The part of the molecule responsible for the color it appears, as a powder or liquid, is called the chromophore. The structure of a molecule can change as the pH changes e.g from acidic to alkaline, and this can include the chromophore. Substances that change color as the pH changes are useful as indicators and can be used in the lab to determine if a substance is acidic or basic or neutral. For example, litmus, a compound found in lichen, is purple under neutral conditions, but turns red in acidic conditions and blue in alkaline conditions.
Why are leaves green?
Plant cells contain chloroplasts, and chloroplasts contain chlorophyll, a green pigment. There are other pigments in plant cells, but much less than the chlorophyll.
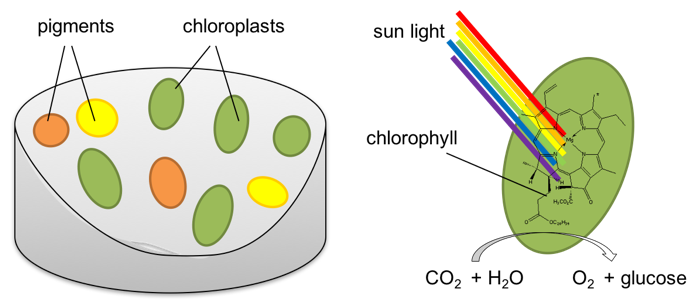
Chlorophyll absorbs light in the blue and red region of the electromagnetic spectrum and plants use the energy from the light they absorb to catalyze a reaction of carbon dioxide and water to form oxygen and glucose, this is called photosynthesis. They release the oxygen into the air and use the glucose, a sugar, as food.