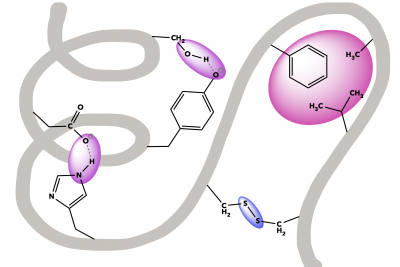
Show the Biochemical Deconstruction of an Egg Sandwich
A scientific exploration of an avocado, egg, and cheese sandwich as delicious examples of lipids, proteins, and carbohydrates
Listed below are some exciting ways in which students and teacher can engage in food exploration from a biochemical perspective. These experiment with facilitate hands-on insight into the nature of specific ingredients to equip student with a well-rounded knowledge of the ways in which chemical processes can alter food composition.
Try with your students...
Cooking Eggs with Acid
Materials

Corner Store Items
- Egg Whites

Lab Chemicals
- 6M HCl
Lab Items
- Glass petri dish, beaker, or jar (glass is best when working with strong acids)
- Micropipette and Micropipette Tips (if you do not have access to a micropipette, you can use a glass Pasteur pipette with a rubber bulb)
Procedure
- Begin with 3.0 g egg white in a small glass beaker (~50 mL beaker works well).
- Use a p1000 micropipette to take 0.5 mL of 6 M HCl, being careful of drips or other hazards that can come from pipetting strong acid.
- If you don’t have a micropipette, you can use a glass Pasteur pipette with a rubber bulb.
- Add the acid DROPWISE into the egg white, swirling slightly to observe the chemical changes happening over time.
- The slow addition of the acid is important for visualizing change.
- Continue to observe the beaker over the next half hour, and eventually you can poke gently with a pipette tip to study the final texture of your egg white.
Purification of Wheat Gluten
Materials

Corner Store Items
- Flour or mix of flours—be sure at least one contains gluten e.g. All-Purpose or Bread flour

General Equipment
- Measuring Cup (1 C)
- Bowl or Bucket

Everyday Items
Running water (or a bucket of water, with the ability to change out the water in the bucket)
Procedure
- Measure 1 C of flour and mix it with 1⁄2 C of water. Mix the flour and water together until a ball of dough begins to form, adding more water if needed. The ball should not feel sticky—if it does, add more flour.
- Knead the ball of dough until it becomes more elastic and shiny on the outside, up to 5 minutes.
- Let the ball sit for at least 10 minutes, which will encourage a higher gluten content.
- Begin to wash away the water soluble carbohydrate component of the dough by putting the dough ball under a gentle stream of water at the tap, or by gently kneading it in a bucket of fresh water. If you are using a bucket of water, change the water as it becomes milky to continue to efficiently remove the carbohydrates.
- Once the water no longer looks milky, you have removed all of the carbohydrates.
Study the properties of your ball of protein. What conclusions can you draw about the effects of their intermolecular interactions?
- If you’d like, you can bake your ball at this stage to see how the heat affects the gluten in the absence of all the other bread-making ingredients.
What conclusions can you draw about how might heat affect gluten protein structure?